Distribution, Botany, and Importance of Anthurium
Within the family Araceae, Anthurium is the largest, most morphologically diverse and complex genus, consisting of approximately 1000 species. Native to Central and South America, members of Anthurium are found at elevations ranging from sea level to 3000m, most commonly in cloud forests at 1500m (Croat 1986). Plants of this herbaceaous perennial monocot are terrestrial or epiphytic. Typical of the amids is the spadix, consisting of a multitude of unobtrusive true flowers supported by a fleshy axil. The protogynous nature of the bisexual flowers in Anthurium favors cross-pollination. The commercial flower is a combination of the spadix and a colorful modified leaf, termed spathe. Attractive foliage of some species makes anthuriums also suitable for leaf harvest and cultivation as a potted plant.
Commercial production has focused on plants derived from two major species, Anthurium andraeanum Linden ex. Andre and A. scherzerianum Schott. (Fig. lA,B). The majority of the plants used in the cut flower industry are thought to be hybrids of A. andraeanum and other species (Madison 1980), and will be referred to as A. andraeanum Hort. Main production areas are Hawaii and The Netherlands, with additional production in other tropical and subtropical regions. The 1991, combined Dutch auctions ranked anthurium 14th of all cut flower sales, with over 20 million stems sold for approximately
$21.5 million (International Floriculture Quarterly Report 1992). Estimates for Dutch auction anthurium sales in 1993 are approximately 37 million stems (International Floriculture Quarterly Report 1994). In Hawaii, anthurium is one of the top cut flowers, with a 1993 farmgate value of sales of $7.5 million for 10.6 million stems sold (Hawaii Agricultural Statistics Service 1994). A. scherzerianum is sold as a flowering potted plant, with main production areas located in Europe. Global production of anthurium hybrids as potted plants has recently increased.
1 Department of Horticulture, University of Hawaii at Manoa, 3190 Maile Way, St. John 102, Honolulu, Hawaii 96822-2279, USA
2 Present address: Department of Horticulture, Purdue University, 1165 Horticultural Building, West Lafayette, Indiana 47906-1165, USA
Biotechnology in Agriculture and Forestry, Vol. 40
High-Tech and Micropropagation VI (ed. by Y.P.S. Bajaj)
© Springer-Verlag Berlin Heidelberg 1997
Micropropagation of Anthurium 15

Fig. 1. A Anthurium andraeanum Hort., cut flower cultivar Nitta. B Anthurium scherzerianum
used in flowering potted plant production. (Photos courtesy H. Kamemoto)
Common Propagation Practices and Need for Micropropagation
Conventional propagation relies upon divisions, cuttings, and in vitro meth ods. Seeds are less commonly used for propagation, as they may produce heterogeneous populations varying in flower color, size, and form.
Division relies on lateral shoots arising from the basal stem portion of the anthurium plant. Some cultivars produce lateral shoots easily while others produce very few. Time until first harvestable flower depends upon initial size of the division, but is generally in terms of months rather than years. Plant growth regulators have been used to stimulate lateral shoot development
within 4 to 6 months after application. A foliar spray of 1000mg/l benzyladenine (BA) applied to intact Ozaki plants resulted in 3.6 lateral shoots per plant, with zero lateral shoots formed on unsprayed plants (Higaki and Rasmussen 1979). Removal of the apical portion of juvenile Mauna Kea plants followed by a 500mg/l GA3 spray increased shoot production from 3.3 shoots (without spray) to an average of 5.8 shoots per sprayed plant (Imamura and Higaki 1988).
Top cuttings, consisting of the uppermost stem with two or three leaves, are removed from plants and rooted in a well-aerated medium. Roots develop within 2 to 3 weeks, with the first flower produced in approximately 6 months. Removal of the top cutting stimulates development of lateral shoots on the mother plant. Basal cuttings, consisting of one or two leafless nodal sections and placed horizontally on medium, produce plants from each node. Although more plants may be generated by basal, rather than top, cuttings, plants take longer to develop and often require 2 to 3 years to reach full production.
In vitro propagation is another method commonly used in anthurium propagation. Plants may be obtained directly from excised apical and lateral buds or indirectly through the differentiation of callus induced from leaf, spathe, and spadix explants.
Rapid clonal propagation is an important use of anthurium tissue culture. In The Netherlands, anthuriums are almost exclusively propagated by culture in vitro (van Doesburg 1991). As recalcitrant genotypes continue to hinder micropropagation of some cultivars, additional in vitro studies should be undertaken.
Disease elimination should be addressed in the propagation of anthuri ums. The bacterial blight caused by Xanthamonas campestris pv. di effenbachiae has been a problem in Hawaii for a decade, and occurs in other anthurium-producing countries such as the Philippines, Jamaica, Tahiti, and Trinidad. Xanthamonas campestris pv. dieffenbachiae may be present in callus and stage II plantlets without visible symptoms, and will not cause medium turbidity in MS-based media that lack coconut water (Norman and Alvarez 1994). Efforts are underway to develop an indexing and certification program for micropropagated anthuriums (Tanabe et al. 1992; Fernandez et al. 1992).
Due to the long breeding cycle of anthuriums, in which the development of a new cultivar may take 8 to 10 years, genetic engineering as a viable breeding aid is currently being investigated at the University of Hawaii for resistance to bacterial blight (Kuehnle et al. 1992b).
Review of In Vitro Studies
Early work on anthurium tissue culture described callus proliferation and subsequent plantlet formation from seeds and young leaf tissue of mature anthurium plants (Pierik et al. 1974). Following this pioneering work, many studies have been conducted for several species and hybrids (reviewed by Geier 1990; Table 1). Different culture media containing modifications of
Table 1. Summary of micropropagation studies in Anthurium. (See also Geier 1990)”
| Anthurium species/hybrids | Explant material | Observations/remarks | Reference |
| A. andraeanum | In vitro plant | Acclimatization of rooted plants | Imamura and Higaki (1981) |
| A. scherzerianum | Spadix fragments | Ploidy variation in callus and regenerated plants | Geier (1982,1988) |
| A. andraeanum | Seed | Multiple plant formation | Tanabe et al. (1989) |
| from seeds germinated in | |||
| vitro | |||
| A. andraeanum | Lamina | Callus and plant regeneration on medium with BA and/or 2,4-D | Lightbourn and Devi Prasad (1990) |
| A. andraeanum | In vitro plant | Certification and indexing | Fernandez et al. (1992) |
| procedure for detection of | Tanabe et al. (1992) | ||
| Xanthamonas campestris pv. diffenbachiae | |||
| A. andraeanum | Etiolated shoot | Protoplast obtained but sustained division not observed | Kuehnle and Nan (1991) |
| A. andraeanum | Lamina and petiole | Shoot and roots from | Kuehnle and Sugii |
| caulogenic callus, genotype effect on regeneration | (1991) | ||
| A. andraeanum | Lamina and spadix | Spadix explants produced more callus formation and plant regeneration than | Singh and Sangama (1991) |
| lamina, with greater ploidy | |||
| uniformity; some | |||
| aneuploids were obtained | |||
| A. andraeanum | In vitro plant | Stage II and stage III plant acclimatization | Tanabe (1991) |
| A. andraeanum | Bud | Effect of genotype on time to first leaf formation | Tanabe et al. (1991) |
| A. andraeanum A. lindenianum A. amnicola A. kamemotoanum | In vitro lamina | Somatic embryos obtained and produced single plants or multiple plant clumps | Kuehnle et al. (1992) |
| A. scherzerianum | Lamina and petiole | Caulogenic callus and plant regeneration | Liu and Xu (1992) |
| A. andraeanum | Bud | Surface disinfestation of buds | Tanabe and Matsumoto (1992) |
| A. cubense | Lamina and petiole | Examined leaf stage, and medium NH,N03 and 2,4-D on callus formation | Warner et al. (1993) |
| A. andraeanum | Lamina, petiole, callus and plantlets | Xanthamonas campestris pv. diffenbachiae may be present in callus and stage II shoots without visible symptoms or medium turbidity | Norman and Alvarez (1994) |
·’ References summarized in Geier ( I990) are not included.
Nitsch (1969) or MS (Murashige and Skoog 1962) basal salts, sugars, other organic components, and growth regulators were described for proliferation and plant regeneration from a variety of tissues. Axillary bud culture (Kunisaki 1980) proved to be another effective micropropagation method. Effects of explant and genotype on regeneration and genetic stability of regen erated plants have also been studied.
Recent studies describe callus, somatic embryogenesis, and protoplast culture. Caulogenic callus was induced from leaf and petiole segments of A. scherzerianum, multiple shoots were obtained, and rooting was induced (Liu and Xu 1992). A. andraeanum Hort. callus and shoot regeneration was re ported by Lightbourn and Devi Prasad (1990), Kuehnle and Sugii (1991), and Singh (1991). Trends in these studies were similar to those reviewed in Geier (1990), where callus is induced on a modified Nitsch (1969) or Pierik et al. (1974) medium supplemented with only 2,4-dichlorophenoxyacetic acid (2,4- D) or a combination of 2,4-D and BA. Plant regeneration was achieved on medium with BA or devoid of growth regulators. A method for somatic embryo induction and conversion to plants was developed for A. andraeanum hybrids using in vitro lamina as explant sources (Kuehnle et al. 1992a). Pre liminary work on anthurium protoplast isolation and culture was presented by Kuehnle and Nan (1991).
Micropropagation
- Establishment of Axenic Cultures
Disinfestation of anthurium tissues can be problematic, with the exception of seeds. Contamination rates usually range from 10 to 20% among leaf explants, 75% in spadix sections (Geier 1990), and 33 to 87% for excised axillary buds (Kunisaki 1980). Due to the protective layers of berry flesh and seed coat, disinfectant may be used at higher concentrations or longer exposure times with fruit and seeds. Slow-growing contaminants are not unusual in anthurium tissues and may appear after the first month of culture. Successfully disinfested explants should show minimal discoloration.
- Seed
Seeds may be disinfested by first soaking the harvested berry in 3% sodium hypochlorite (NaOCl; e.g., 57.7% Clorox) for 15min, followed by soaking excised seeds in 1 % NaOCl for 20min (Pierik et al. 1974). Each soak is accompanied by a 30-min rinse with several volumes of sterile water. The seed coat is then removed, and the explant, consisting of the embryo and en dosperm, is cultured on appropriate medium (Pierik et al. 1974). Calcium hypochlorite can be substituted for sodium hypochlorite (Rosario and Lapitan
1981). Successful disinfestation has also been achieved by one soak of excised seed in 2.6% NaOCl for 5min (Zens and Zimmer 1988) or LD disinfectant (Alcide Corp., Norwalk, Connecticut) at 1 part activator: 1 part base: 10 parts water for 30min (Tanabe et al. 1989). Presence of a gelatinous or sticky substance often hinders the handling of the anthurium seeds with standard tissue culture tools. Although it is not usually present in seeds disinfested with NaOCl, removal of this substance is possible with a 13% sodium carbamate solution (Maurer and Brandes 1979). Seeds or excised embryos germinate within 4 weeks, with proliferation of callus usually occurring within 12 to 16 weeks (Rosario and Lapitan 1981).
- Leaf, Spathe, and Spadix
Lamina, petiole, and spathe sections are generally disinfested by an initial dip in 70 to 95% alcohol, followed by a 10- to 30-min soak in 1.5 to 3% NaOCl. An alternative method uses a 5-min soak in 0.1% mecuric chloride solution with
0.25 ml/1 Tween 20 in place of NaOCl (Eapen and Rao 1985). Similar methods are used for spadix explants, in which first the spathe surrounding the young spadix is disinfested, followed by disinfestation of the spadix proper (Geier 1982).
Unprotected or screenhouse cultivation of plants in subtropical and tropi cal areas is conducive to high contamination rates for field-grown material. In Jamaica, use of a 70% alcohol dip for 45s and a 1.25% NaOCl soak for 15min resulted in up to 70% contamination of leaf explants. Contamination was reduced to 10% by a presterilization soak in the fungicide Benlate (Dupont) (Lightbourn and Devi Prasad 1990). Axenic leaf blade and petiole cultures have been obtained using an initial 10-min soak in 0.14% Physan 20 (Maril Products Inc., Tustin, California) followed by consecutive soaks of 30min in 0.53%, then 0.27%, NaOCl with one drop Tween 201100ml (Kuehnle and Sugii 1991); contamination rates of 5% are routinely achieved (T. Matsumoto, unpubl.).
While leaf callus produced from plants infected by Xanthomonas campestris pv. dieffenbachiae was found to be axenic, it should be noted that this bacterial pathogen can be harbored asymptomatically in inoculated callus and shoots for 4 months to 1 year (Norman and Alvarez 1994).
- Axillary Buds
Initial reports record a contamination rate of 33% using two soaks for 20 and 45min in 0.53 and 0.27% NaOCl solution with Tween 20, respectively, with removal of bud scales (Kunisaki 1980). Reduction of exposure time to the disinfestant is possible through use of LD and Exspor disinfectants supple mented with 35% isopropyl alcohol (Alcide Corp., Norwalk, Connecticut; Tanabe and Matsumoto 1992).
Methods for Culture Initiation and Plant Multiplication
Enhanced axillary branching from microcuttings of in vitro shoots is used to multiply plants to the quantities desired. The initial shoots are obtained from a variety of sources, namely callus cultures, axillary buds, and somatic em bryos. Several factors affect the success of anthurium micropropagation, and some aberrations have been described among regenerated plants.
3.2.l Callus
Callusing is used to initiate in vitro cultures of field material from which shoots for subsequent multiplication are derived (Fig. 2). This method is commonly used in The Netherlands and numerous reports have been published. Organogenic callus and plant regeneration have been achieved using seeds, embryos, and explant material of leaf lamina, petiole, spadix, spathe, and etiolated shoots (Geier 1990; Lightbourn and Devi Prasad 1990; Kuehnle and Sugii 1991; Liu and Xu 1992). In general, callus induction and proliferation are favored under dark conditions by addition of an auxin, usually 2,4-D, and a cytokinin, usually BA, to solid or liquid medium. Shoot proliferation from callus is stimulated with the removal of auxin from the medium, re duction of ammoniacal nitrogen, and increased light. Cytokinins such as 2- isopentenyladenine (2iP), BA, or kinetin may, in some cases, be required for shoot formation.
The soft tissue of newly unfolded leaves is successfully used for laminar explants. Explants of fully expanded leaves should include a major vein with
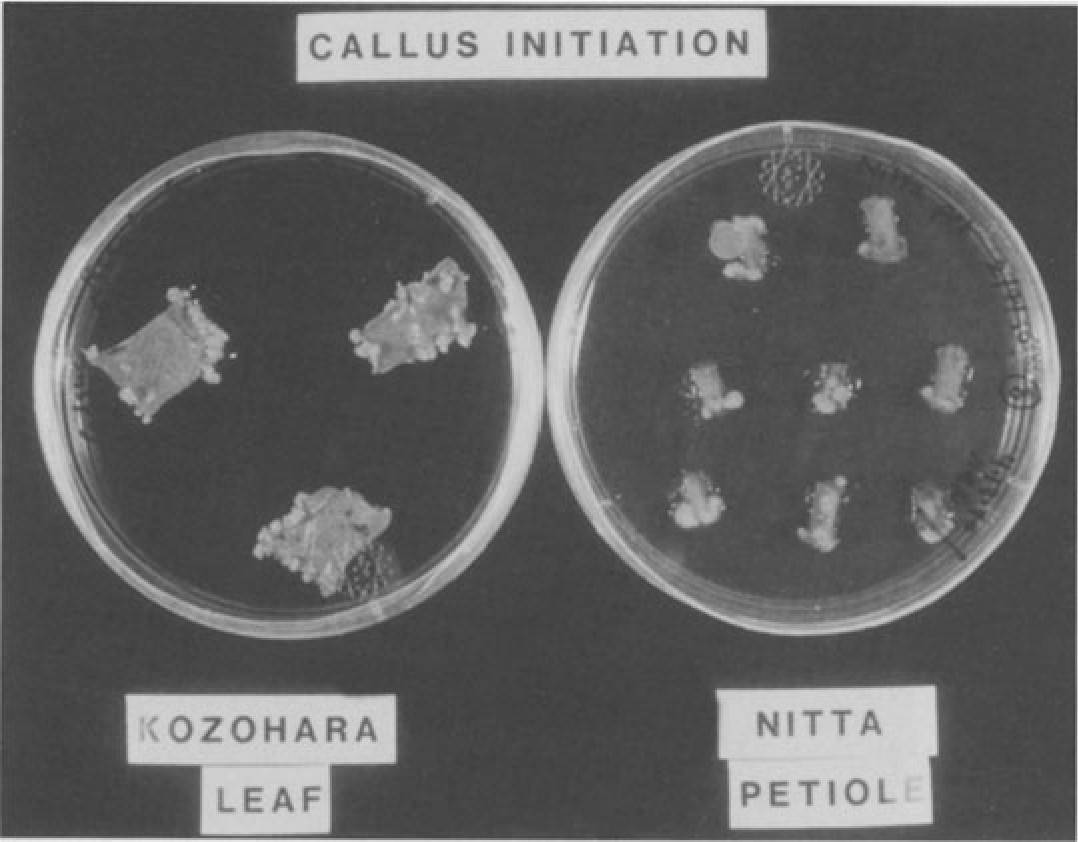
Fig. 2. Callus initiated from Kozohara lamina and Nitta petiole sections on modified Pierik medium with 0.36µM 2,4-D and 4.4µM BA with 0.18% Gelritc in the dark
Micropropagation of Anthurium 21
vascular tissue for improved proliferation (Finnie and van Staden 1986). It is suggested that young, unlignified leaves about one-half to two-thirds of the final length are most useful for A. scherzerianum (Geier 1990). Optimum regeneration in A. andraeanum occurs if leaves are harvested 1 day after they are fully expanded. Lamina sections show signs of proliferation as early as 2 to 4 weeks (Finnie and van Staden 1986) up to 12 to 16 weeks (Lightbourn and Devi Prasad 1990).
- Axillary Bud
Direct shoot formation from excised buds was reported by Kunisaki (1980). This method establishes initial shoot cultures without an intervening callus phase, and thus may reduce the possibility of somaclonal variation and abnor mal plant recovery, but at the expense of rapid propagation. According to Kunisaki (1980) and later adaptations (J. Kunisaki, pers. comm.) five to ten lateral buds are obtained from the stem of an anthurium plant (Fig. 3A), surface sterilized, and trimmed to 2 mm at the base. Shoot formation is encour-
A 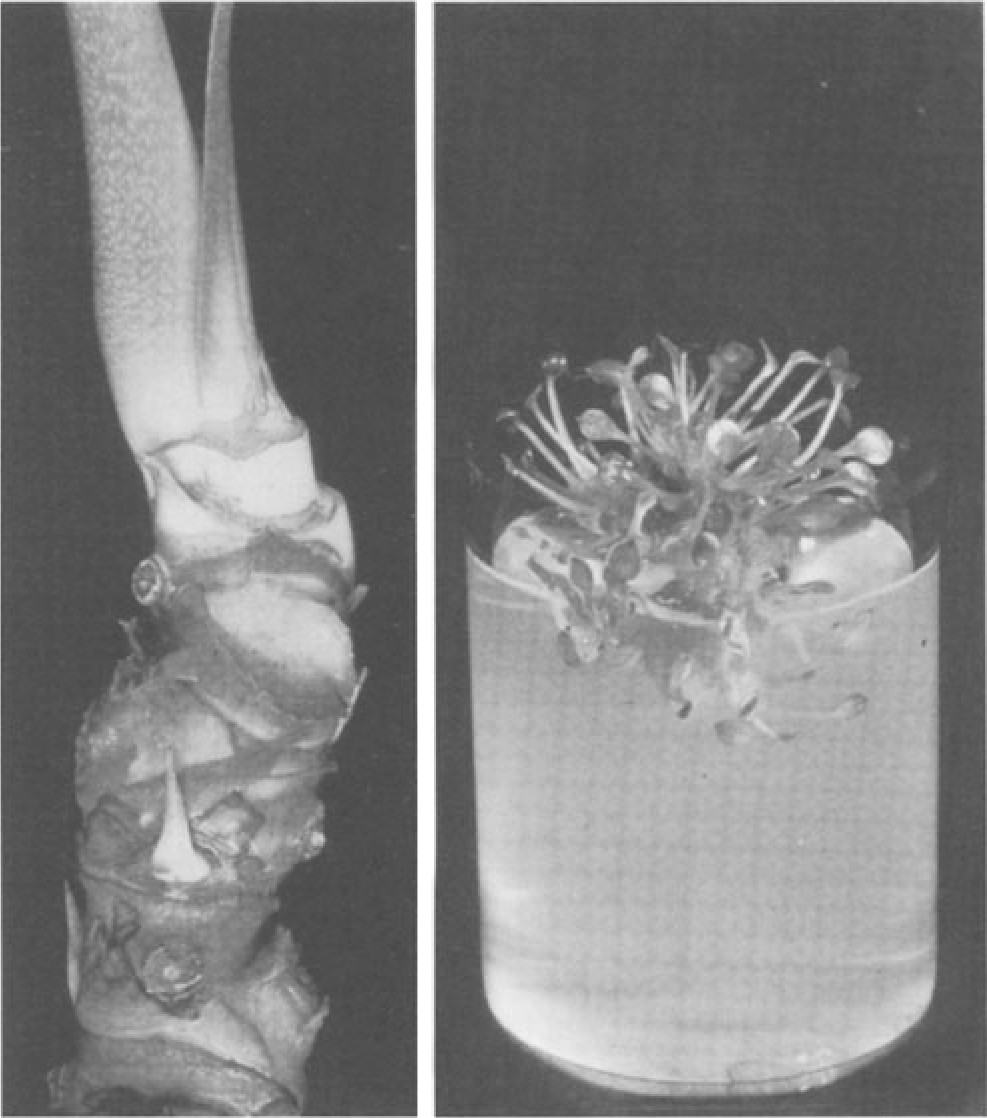 B
B
Fig. 3A,B. Axillary bud culture. A Stem section of mother plant, stripped of leaf sheaths to expose lateral buds. B Multiple shoot formation from stem sections of plants grown from axillary buds on modified half-strength MS medium with 2% sucrose and 0.89µMBA. (Photos courtesy J. Kunisaki)
aged in a liquid modified MS medium consisting of MS salts at 3/8 strength, 15% coconut water, and 2% sucrose. After a single elongated shoot develops from each bud, usually within 12 to 18 months, a top cutting consisting of the apex and two or more leaves is cultured on a filter paper bridge in similar medium. For multiple shoot formation, basal portions of the remaining stem are placed on solid medium, or on a shaker in liquid medium, supplemented with 0.2 mg/I BA for a maximum of 2 months (Fig. 3B). Top cuttings taken from the multiple shoots are placed on medium used for initial shoot forma tion and solidified with 0.18% Gelrite for shoot growth and root formation. The basal explants are subcultured again to solid medium lacking BA for additional shoot formation. Once shoots form, top cuttings are taken once again and the remaining bases discarded.
- Somatic Embryogenesis
Somatic embryogenesis and subsequent plant regeneration have been achieved in A. andraeanum hybrids. Whole lamina explants were harvested from in vitro-grown plants and plated on a modified half-strength MS medium with 2% sucrose, 1% glucose supplemented with 1 to 4mg/12,4-D, and 0.33 to 1 mg/I kinetin. Induction of embryos (Fig. 4) and proliferation of secondary embryos occurred under darkness. Conversion and maturation occurred on the same basal medium plus 2% sucrose, 0.2mg/l BA, and 0.18% Gelrite under a 16-h photoperiod (Kuehnle et al. 1992a). These plantlets can serve as a source of nodal microcuttings for subsequent multiplication.
In other reports, somatic embryogenesis in spadix callus of Anthurium scherzerianum was induced by lowering ammonium nitrate to 1.25 mM
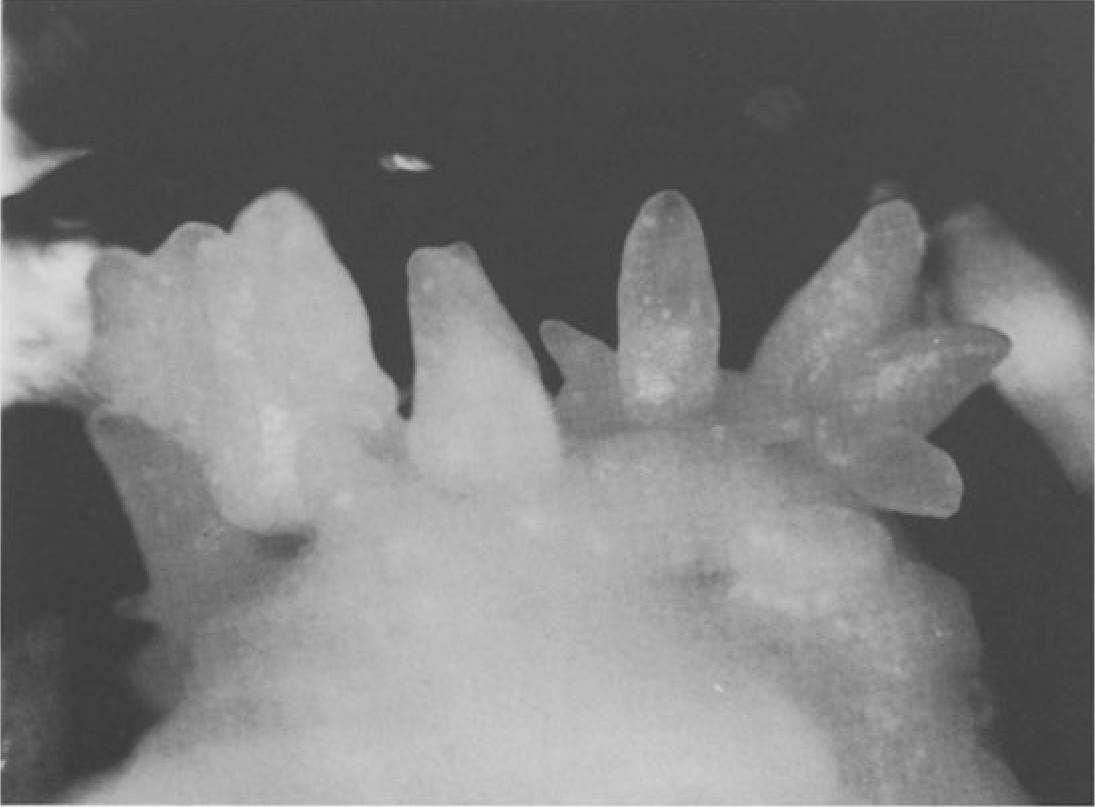
Fig. 4. Somatic embryos of Nitta cultured in the dark on modified half-strength MS medium with 2% sucrose, 1% glucose, 18µM 2,4-D, and 2.3µM kinetin
Micropropagation of Anthurium 23
NH4N03 in a Nitsch medium with 4.44µM BA and 0.45µM 2,4-D (Geier 1982). Somatic embryos germinated into bipolar structures and multiple plantlet clumps. However, these somatic embryos occurred sporadically (Geier I 990). Petioles from in vitro-grown plants of Lady Jane (A. andraeanum X A. antioquiense) plated on a modified MS medium with 2µM 6- benzylaminopurine (BAP), 2µM zeatin, and lµM 2iP, also appeared to pro duce somatic embryos (F.J. Novak, pers. comm.). Cultures were kept in the dark for 2 to 3 months at 28 °C and maintained on the same medium, either solid or liquid. Shoots were regenerated on a medium containing 0.5 µM IBA (indolebutyric acid).
- Influence of Genotype and Selection of Explant
Genotype plays an important role in the multiplication and regeneration of anthuriums (Pierik et al. 1974). As with other crops, examples of variation among genotypes exist for anthurium callus formation (Geier 1990; Light bourn and Devi Prasad 1990; Kuehnle and Sugii 1991; Liu and Xu 1992) and somatic embryogenesis (Kuehnle et al. 1992a). Differences in cultivar re sponses also apply to enhanced axillary branching, with the period of first leaf emergence ranging from 13 weeks for Hawaiian Butterfly to 38 weeks for Fuji Pink (Tanabe et al. 1991).
Selection of the explant is often dependent upon the material available and the objective of study. Limited number of axillary buds are available per individual mother plant, and recovery of the mother plant following removal of a large stem section for excision of axillary buds may take over one year. In contrast, greater amounts of propagative material are generally available for other methods, and removal of a leaf, spathe, or spadix will not cause substan tial injury to a mother plant.
For clonal propagation, the use of seeds is highly discouraged due to the high variability in offspring from cross-pollinations. However, seed culture has been proposed as a breeding aid applicable to limited number of crosses or those with few resulting seeds (Tanabe et al. 1989). Clones are produced in vitro by promotion of multiple shoot formation from germinating seeds. Clones of each seed are evaluated by the breeder in the field; corresponding clones are retained in vitro. If and when the selection of a promising cultivar is made, propagules are already available in vitro for subsequent multiplication.
- Rooting
Shoots will spontaneously root in culture under light conditions following removal or depletion of growth regulators, notably cytokinins, in the nutrient medium. If rooting does not readily occur, medium salts may be reduced to half the original strength and the solidifying agent may be reduced or elimi nated. Some propagators include charcoal as a darkening agent in the rooting medium (N. van der Knaap, pers. comm.).
- Acclimatization
Anthurium microcuttings rooted in vitro (termed stage III) prior to transfer to the greenhouse are generally hardier than unrooted propagules (stage II), but may be less cost-effective for the micropropagator or grower. Retail price per stage II microcutting is currently 20% less than stage III material for major labs in the USA. Success of acclimatization at stage II is often dependent on the particular cultivar. Many labs also sell acclimatized plants (Stage IV). These often ensure a better unit price per plant for the laboratory and increase survivability of plants for the buyer. Plants at any of these stages are suscep tible to bacterial blight, rain and slug damage, and salt burn from use of undiluted complete fertilizer.
Stage II and stage III microcuttings should be carefully removed from the culture vessels and all remaining agar rinsed off. Stage II shoots preferably should contain the apex plus two or three leaves prior to transplantation ex vitro. Microcuttings are placed into a sterile, premoistened medium such as Oasis Root Cube, rockwool, or vermiculite/perlite combinations. Plants are kept in high humidity under 80% shade or 1500 to 2000 foot candles for approximately 2 months (Tanabe 1991). Stage III plantlets may be treated with a fungicide as a preplant soak or postplant drench (Imamura and Higaki 1981). Plants with intact roots are transplanted into a variety of media includ ing Oasis or rockwool plugs, shredded tree fern fiber, or mixes of soil amend ments such as composted shredded bark: no. 2 perlite (3:1 mix) under 80% shade with high humidity.
In large-scale commercial productions, misting or fogging systems are often employed to provide high humidity conditions conducive for further plant growth. Other methods include use of humidity tents for multiple flats (Fig. SA) or clear plastic domes for individual flats (Fig. SB). Plants are monitored and watered to prevent desiccation. The plastic cover should be removed in incremental stages, to gradually reduce the humidity (Tanabe 1991). Plants should continue to be protected from rain until large enough for transplant into beds or pots (Higaki et al. 1994).
- Somaclonal Variation
Aberrations are occasionally observed among micropropagated plants. Al though ploidy variation was found in callus of A. scherzerianum, carry-over to the regenerated plant was rare, based on stoma length, chromosome counts, and cytophotometry (Geier 1988, 1990).
Another source of variation may relate to the proliferation method used. Somaclonal variation was documented for plants obtained both from year old callus pieces and from plants initiated by axillary bud culture and micropropagated long-term by enhanced axillary branching (Kuehnle and Sugii 1992; Kuehnle and Kuanprasert, unpubl.). Using 3 diverse genotypes, approximately 520 plants micropropagated via long-term callus culture (12 to 13 months) and 120 plants micropropagated via nodal cuttings, over a 3- to 4- year period, were grown to maturity and evaluated. Plants were grouped into
Micropropagation of Anthurium 25
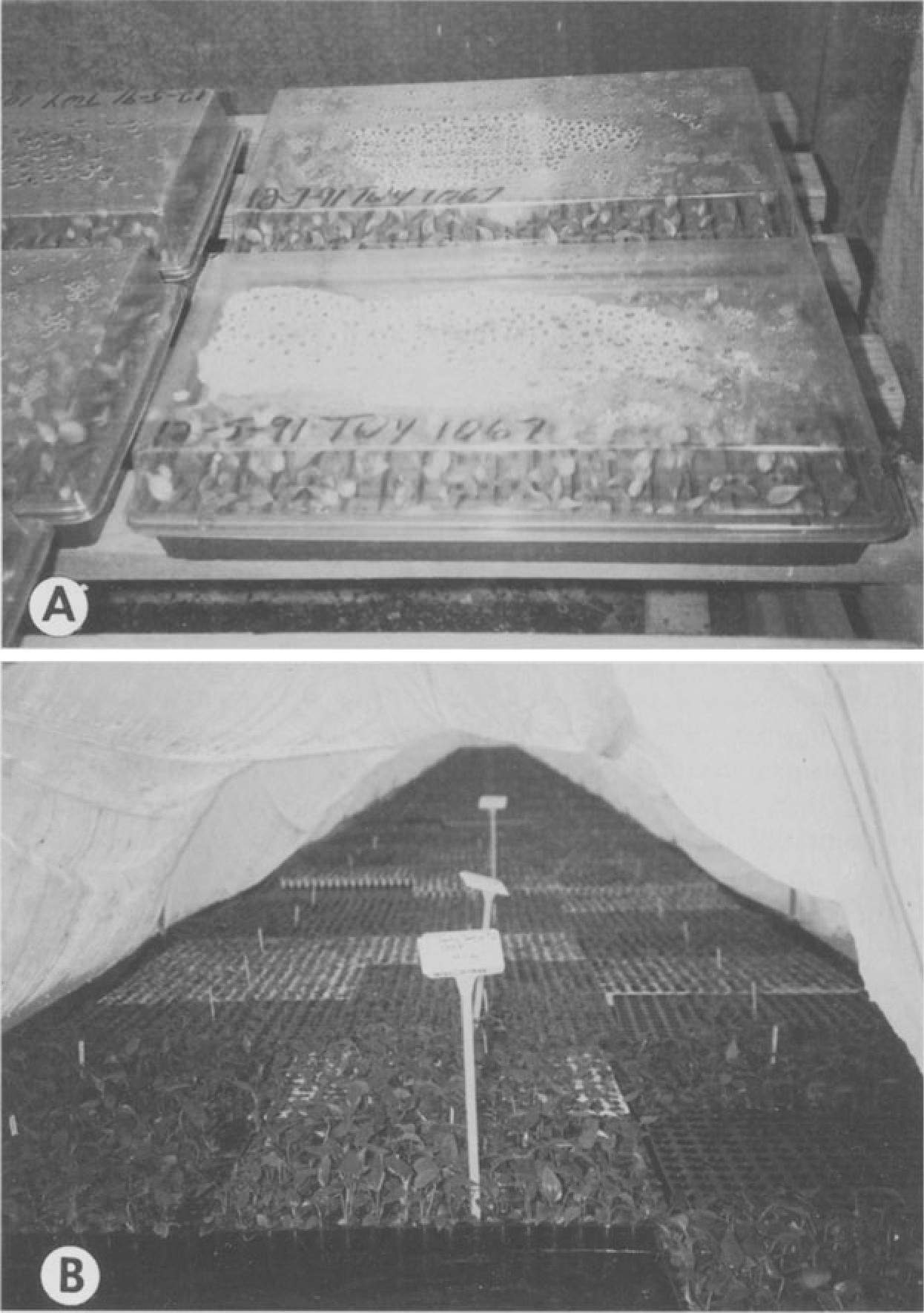
Fig. SA,B. Acclimatization: A Stage III anthuriums transplanted ex vitro into trays containing Oasis rooting cubes and covered with plastic hoods. B Humidity tent used to cover mass plantings of anthurium
those arising from the same callus piece immediately prior to shoot regenera tion, or those arising from the same nodal cuttings in the last subcultures prior to planting in the greenhouse.
Both sets of plants experienced stunting or shortening of internodes for all three genotypes. Shortened internodes occurred among 19 to 30% of the callus groups, but infrequently within a group, so that as few as 4% of the total
plants from a genotype may have had reduced stature. Plants from nodal cuttings showed a higher rate of stunting (25 to 43% of related groups), but no other aberrations. Among callus-derived plants, leaf shape abnormalities, leaf variegation or ploidy changes were detected in 0.8 to 2% of the total plants, corresponding to 3 to 8% of the original callus clumps put on plant regenera tion medium; frequency varied among genotypes.
Based on these findings, it is suggested that callus proliferation be re stricted to a few months prior to plant regeneration and that new stock plants for enhanced axillary branching, derived from callus or from axillary buds, be re-initiated at regular intervals of less than 3 years; every 6 to 12 months is practiced in The Netherlands.
- Commercial Aspects (Table 2)
Among commercial tissue culture laboratories in 14 Western European coun tries surveyed in 1988, Anthurium ranked 14th among the most frequently propagated genera, with three countries producing more than 100000 anthu rium plants each (Pierik 1991). The Netherlands, which accounts for 29% of the West European production, micropropagated over 0.5 million plants of A. andraeanum in 1988, placing A. andraeanum second after Gerbera in quantity of plants produced for the cut flower industry. For the potted plant market, A. scherzerianum ranked fourth, after Nephrolepis, Saintpaulia, and Ficus, with
1.7 million plants produced in vitro in The Netherlands (Pierik 1991). Figures for the quantity of plants micropropagated from 1988 to 1990 have been compiled and are shown in Table 2 (R.L.M. Pierik, pers. comm.). In The Netherlands, initial multiplication relies largely upon callus culture.
To estimate the extent of Anthurium micropropagation in the USA, a survey was sent to major commercial tissue culture laboratories in Hawaii and the continental USA. Of seven laboratories currently producing over 10000 anthuriums per year, an estimated 3.8 million plants were micropropagated in 1993, with three of these labs producing 90% of the supply with over 1 million plants each. The majority of plants were for use in cut flower and flowering potted plant production; fewer were produced for the foliage industry. A variety of A. andraeanum Hort., A. scherzerianum, and hybrids with A. amnicola and A. antioquiense are produced for the flowering potted plant market. All seven laboratories rely primarily on axillary and apical bud culture
Table 2. Quantity of micropropagated anthurium plants in The Netherlands, 1988 to 1990. (Courtesy R.L.M. Picrik, Agricultural University, Wageningen, The Netherlands)
| 1988 | 1989 | 1990 | |
| A. andraeanum | 509300 | 919000 | 1516500 |
| A. scherzerianum | 1684624 | 2454400 | 4157750 |
| Total plants | 2193924 | 3373400 | 5674250 |
for culture establishment, followed by axillary branching for multiplication; two of seven (29%) labs use callus or somatic embryogenesis as a supplemen tal form of culture establishment. The vast majority of plants sold are derived from initial bud culture. Micropropagated anthurium plants are sold by 71% of the laboratories as stage III or stage IV; 29% of the labs also offer stage II microcuttings. In terms of total plants sold, stages III and IV are slightly more popular, with an estimated 35% stage III, 37% stage IV, and 28% stage II sold by the seven laboratories.
Summary and Conclusions
Methods for fast clonal propagation are essential to fulfill growers’ demands for anthurium plants worldwide. Heterogeneous seed progeny and slow meth ods of field propagation are unsuitable for generating the large quantities needed. Efficiency of callus culture has been greatly improved for many geno types since this system was first described for anthurium in 1974 by Pierik et al. Alternative methods, such as axillary bud culture and somatic embryogenesis, have also been developed. Currently, all three methods are commercially used for micropropagation of cut flower and potted plant anthuriums. Slow re sponse of certain genotypes is still a problem despite modifications to the culture medium. Mass propagation linked with disease-indexing programs are currently being investigated, and will be beneficial to production areas plagued with the bacterial blight. In the future, in vitro regeneration of transgenic plants might play an important role in breeding for disease resistance and other desirable qualitative traits.
- Protocol
Axillary Bud
- Excise buds with a 1-cm base from a stem which has been washed with soap, soaked in 0.53% NaOCI for 5 min, and air-dried for 2-3 days.
- Soak in 0.53% NaOCI for 30 min, rinse, then remove two to three leaf sheaths and cut to 2 mm.
- Soak again in disinfestation solution for 5 min and rinse in sterile water.
- Place bud in liquid half-strength MS medium with 2% sucrose, 15% coconut water in culture tube on rotary drum at 0.2 rpm, under light, until shoot elongates with leaves.
- Use basal stem cutting for enhanced axillary branching on half-strength MS medium with 2% sucrose and 0.2mg/I BA under light; shoots are rooted on hormone-free medium in light.
Callus
- Surface sterilize petiole and lamina sections in 0.14% Physan 20 for lOmin, 0.53% NaOCI for 30min, and 0.26% NaOCI for 30min, then rinse in sterile water.
- Plate on solid modified MS medium containing 1 mg/I BA and 0.08-0.1 mg/I 2,4-D in dark.
- After several months, explants with callus are moved into light for shoot proliferation on BA medium.
- Cuttings are taken for enhanced axillary branching and rooting, as in 5.1.5, above.
References
Croat T (1986) The distribution of Anthurium (Araceae) in Mexico, Middle America and Panama.
Selbyana 9: 94-99
Eapen S, Rao PS (1985) Regeneration of plants from callus cultures of Anthurium patulum. Curr Sci 54: 284-286
Fernandez JA, Tanabe M, Crane S, Wolff W, Moriyasu P (1992) Anthurium in vitro triple indexing II. In: Delate KM, Tome CHM (eds) Proc 5th Hawaii Anthurium Industry Conf. Hawaii Institute of Tropical Agriculture and Human Resources, HIT AHR 02.02.94, Univer sity of Hawaii, Honolulu, p 19
Finnie JF, van Staden J (1986) In vitro culture of Anthurium andraeanum. S Afr J Bot 52: 343-346 Geier T (1982) Morphogenesis and plant regeneration from spadix fragments of Anthurium scherzerianum cultivated in vitro. In: Fujiwara A (ed) Plant tissue culture 1982. Maruzen,
Tokyo, pp 137-138
Geier T (1988) Ploidy variation in callus and regenerated plants of Anthurium scherzerianum
Schott. Acta Hortic 226: 293-298
Geier T (1990) Anthurium. In: Ammirato PV, Evans DA, Sharp WR, Bajaj YPS (eds) Handbook of plant cell and tissue culture, vol 5. Ornamental species. McGraw-Hill, New York, pp 228- 252
Hawaii Agricultural Statistics Service (1994) Hawaii flowers and nursery products, annual sum mary. Hawaii Dep Agric, US Dep Agric, Honolulu, Hawaii
Higaki T, Rasmussen HP (1979) Chemical induction of adventitious shoots in anthurium.
HortScience 14: 64-65
Higaki T, Lichty JS, Moniz D (1994) Anthurium culture in Hawaii. HIT AHR Research Extension Series 152, University of Hawaii, Honolulu
Imamura J, Higaki T (1981) Transfer of tissue-cultured anthurium plantlets from flask to natural conditions. Hortic Digest 61: 1-2
Imamura J, Higaki T (1988) Effect of GA3 and BA on lateral shoot production on Anthurium.
HortScience 23: 353-354
International Floriculture Quarterly Report (1992) Cut flower 1991 rankings, vol 3. Pathfast Publishing, Essex, pp 56-62
International Floriculture Quarterly Report (1994) Cut flower and pot plant prices through the Dutch auctions, vol 4. Pathfast Publishing, Essex, pp 63, 77-78
Kuehnle AR, Nan GL (1991) Isolation and culture of protoplasts from two tropical monocots,
Anthurium and Dendrobium orchid. Physiol Plant 82: A7
Kuehnle AR, Sugii N (1991) Callus induction and plantlet regeneration of Hawaiian anthuriums.
HortScience 26: 919-921
Kuehnle AR, Sugii N (1992) Update on somatic embryogenesis research. In: Delate KM, Tome CHM (eds) Proc 5th Hawaii Anthurium Industry Conf. Hawaii Institute of Tropical Agricul ture and Human Resources, HIT AHR 02.02.94, University of Hawaii, Honolulu, pp 15-16
Kuehnle AR, Chen FC, Sugii N (1992a) Somatic embryogenesis and plant regeneration in Anthu rium andraeanum hybrids. Plant Cell Rep 11: 438-442
Kuehnle AR, Chen FC, Sugii N, Jaynes J, Norman D, Alvarez A (1992b) Engineering blight resistance in Anthurium: a progress report. In: Delate KM, Tome CHM (eds) Proc 5th Hawaii Anthurium Industry Conf. Hawaii Institute of Tropical Agriculture and Human Resources, HIT AHR 02.02.94, University of Hawaii, Honolulu, pp 17-18
Kunisaki JT (1980) In vitro propagation of Anthurium andreanum Lind. HortScience 15: 508-509 Lightbourn GJ, Devi Prasad PV (1990) In vitro techniques for rapid multiplication of four
varieties of Anthurium andraeanum in Jamaica. Proc Interam Soc Trop Hort 34: 3-5
Liu CM, Xu ZH (1992) An efficient procedure for micropropagation of Anthurium scherzerianum
Schott (flamingo flower). Chin J Bot 4: 49-55
Madison M (1980) Aroid profile no. 6: Anthurium andreanum. Aroideana 3: 58-60
Maurer M, Brandes S (1979) Die Extraktion der Samen von Anthurium scherzerianum-Hybriden.
Gartenbauwissenschaft 44: 71-73
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473-497
Nitsch JP (1969) Experimental androgenesis in Nicotiana. Phytomorphology 19: 389-404 Norman D, Alvarez A (1994) Latent infections of in vitro anthurium caused by Xanthomonas
campestris pv. dieffenbachiae. Plant Cell Tissue Organ Cult 39: 55-61
Pierik RLM (1991) Commercial micropropagation in Western Europe and Israel. In: Debergh PC, Zimmerman RH (eds) Micropropagation. Kluwer, Dordrecht, pp 155-165
Pierik RLM, Steegmans HHM, van der Meys JAJ (1974) Plantlet formation in callus tissues of
Anthurium andraeanum Lind. Sci Hortic 2: 193-198
Rosario TL, Lapitan LA (1981) Callus and plantlet formation in Anthurium andraeanum Lind.
Phillipp Agric 64: 197-202
Singh F, Sangama (1991) Micropropagation and plant conformity in Anthurium andraeanum. In: Prakash J, Pierik RLM (eds) Horticulture – new technologies and applications. Kluwer, Dordrecht, pp 201-204
Tanabe M (1991) In vitro plant acclimatization. In: Leonhardt K, Evans D, Halloran J (eds) Hawaii tropical cut flower industry conference: growing in the 90’s. Hawaii Institute of Tropi cal Agriculture and Human Resources, HITAHR Res Ext Series 124, University of Hawaii, Honolulu, pp 100-101
Tanabe M, Matsumoto T (1992) Anthurium explant surface disinfestation. In: Delate KM, Tome CHM (eds) Proc 5th Hawaii Anthurium Industry Conf. Hawaii Institute of Tropical Agricul ture and Human Resources, HITAHR 02.02.94, University of Hawaii, Honolulu, pp 10-11
Tanabe M, Keolanui R, Campbell J (1989) Anthurium seed tissue culture. In: Fernandez JA, Nishijima WT (eds) Proc 2nd Anthurium Blight Conf. Hawaii Institute of Tropical Agriculture and Human Resources, HITAHR 03.10.89, University of Hawaii, Honolulu, pp 52-53
Tanabe M, Arakawa C, Matsumoto T, Tanaka R, English J (1991) Anthurium in vitro propaga tion. In: Alvarez AM, Deardorff DC, Wadsworth KB (eds) Proc 4th Hawaii Anthurium industry conf. Hawaii Institute of Tropical Agriculture and Human Resources, HITAHR 06.18.91, University of Hawaii, Honolulu, pp 4-6
Tanabe M, Fernandez J, Moriyasu P, Crane S, WolffW, Liu RW (1992) Anthurium in vitro triple indexing. In: Delate KM, Tome CHM (eds) Proc 5th Hawaii Anthurium Industry Conf. Hawaii Institute of Tropical Agriculture and Human Resources, HITAHR 02.02.94, Univer sity of Hawaii, Honolulu, pp 8-9
van Doesburg J (1991) What it takes to be successful: promotion, an essential factor in marketing. In: Leonhardt K, Evans D, Halloran J (eds) Hawaii tropical cut flower industry conference: growing in the 90’s. Hawaii Institute of Tropical Agriculture and Human Resources, HITAHR Res Ext Series 124, University of Hawaii, Honolulu, pp 22-31
Warner J, Herrera J, Guevara E (1993) Morphogenesis in vitro de Anthurium cubense (Araceae).
Rev Biol Trop 41: 455-460
Zens A, Zimmer K (1988) Development of new cultivars of Anthurium scherzerianum Schott through in vitro propagation III. In vitro regeneration from leaf explants of clones. Gartenbauwissenschaft 53: 170-173

