INTRODUCTION
The inception of science of plant tissue culture takes its roots from the discovery of cell followed by propounding of cell theory. In 1838, Schleiden and Schwann proposed that cell is the basic structural unit of all living organisms. They visualized that cell is capable of autonomy and therefore it should be possible for each cell if given an environment to regenerate into whole plant. Based on this premise, in 1902, a German physiologist, Gottlieb Haberlandt for the first time attempted to culture isolated single palisade cells from leaves in knop’s salt solution enriched with sucrose. The cells remained alive for up to one month, increased in size, accumulated starch but failed to divide. Though he was unsuccessful but laid down the foundation of tissue culture technology for which he is regarded as the “father” of plant tissue culture. Plant tissue culture is the in vitro aseptic culture of cells, tissues, organs or whole plant under controlled nutritional and environmental conditions often to produce the clones of plants (Thorpe, 2007). The resultant clones are true-to type of the selected genotype. The controlled conditions provide the culture an environment conducive for their growth and multiplication. These conditions include proper supply of nutrients, pH medium, adequate temperature and proper gaseous and liquid environment. Plant tissue culture technology is being widely used for large scale plant multiplication. Apart from their use as a tool of research, plant tissue culture techniques have in recent years, played an important role in the area of plant propagation, disease elimination, plant improvement and production of secondary metabolites, using small pieces of tissue (named explants) to produce hundreds and thousands of plants in a continuous process. A single explant can be multiplied into several thousand plants in relatively short time period and space under controlled conditions, irrespective of the season and weather on a year round basis (Akin-Idowu et al, 2009). Endangered, threatened and rare species have successfully been grown and conserved by micropropagation because of high coefficient of multiplication within less space. In addition, it is also considered to be the most viable technology for crop improvement by the production of somaclonal and gametoclonal variants. The micropropagation technology has a vast potential to produce plants of superior quality, isolation of useful variants in well-adapted high yielding genotypes with better disease resistance and stress tolerance capacities (Brown and Thorpe, 1995; Suman et al, 2012). Certain type of callus cultures give rise to clones that have inheritable characteristics different from those of parent plants due to the possibility of occurrence of somaclonal variability
(George, 1993; Suman et al, 2015) which leads to the development of commercially important improved varieties. Commercial production of plants through micropropagation techniques has several advantages over the traditional methods of propagation through seed, cutting, grafting and air-layering etc. It is rapid propagation processes that can lead to the production of virus free plants (Garcia-Gonzales et al, 2010). Meristem tip culture of banana plants devoid from banana bunchy top virus (BBTV) and brome mosaic virus (BMV) were produced (El-Dougdoug and El-Shamy, 2011). Higher yields have been obtained by culturing pathogen free germplasm in vitro. Increase in yield up to 150% of virus-free potatoes was obtained in controlled conditions (Singh, 1992).
Agricultural diversification to meet our future needs call for the adoption of new technologies in agriculture. Utilization of the best cultural practices, fertilization, pest control measures will not give the necessary results without the use of best planting material. Now a day, tissue culture is adopted as a viable horticultural propagation method which has revolutionized the horticultural industry. Mass propagation and the establishment of disease free stock material is accomplished by use of this technique (Bachraz, 1998). Tissue culture is a method of vegetative propagation based on biotechnology. The plants are derived from stem, root or leaf tissues and the technology generally aids in mass production of desired crop varieties. Tissue culture is also useful in regeneration of genetically modified cells into whole plants as well as in embryo rescue techniques (Bio Vision, 2008). Ilan and Workafes (2011) have put on record the advantages and disadvantage of this method as that controlled environment and controlled development of the plants enable very rapid multiplication rate and clean conditions for plant development that produce micro-plants free of many pests and diseases. The small size of the propagated plants saves nursery space and plant transport costs. Reproducing the planting materials of vegetatively propagated crops presents complex problems because of lack of knowledge of phyto-sanitary measures and quarantine issues related to safe movement of germplasm, plants and planting material across national borders, lack of consistent supplies of good quality planting material, variable demand for clean planting material, bulkiness and perishability of planting materials and use of traditional varietal mixtures, including local varieties (Wambugu and Kiome, 2001). The prime basic objective of this review is to describe the tissue culture techniques, various developments, present and future trends and its application in various fields with particular reference of banana.
TECHNOLOGICAL INNOVATIONS IN PLANT TISSUE CULTURE
Know-How
In the process of plant cell culture, plant tissues and organs are grown in vitro on artificial media, under aseptic and controlled environment. The technique depends mainly on the concept of totipotentiality of plant cells which refers to the ability of a single cell to express the full genome by cell division (Haberlandt, 1902). Along with the totipotent potential of plant cell, the capacity of cells to alter their metabolism, growth and development is also equally important and crucial to regenerate the entire plant (Thorpe, 2007). Plant tissue culture medium contains all the nutrients required for the normal growth and development of plants. It is mainly composed of macronutrients, micronutrients, vitamins, other organic components, plant growth regulators, carbon source and some gelling agents in case of solid medium (Murashige and Skoog, 1962). Murashige and Skoog medium (MS medium) is most extensively used for the vegetative propagation of many plant species in vitro. The pH of the media is also important that affects both the growth of plants and activity of plant growth regulators. It is adjusted to the value between 5.4 – 5.8. Both the solid and liquid medium can be used for culturing. The composition of the medium, particularly the plant hormones and the nitrogen source has profound effects on the response of the initial explant. Plant growth regulators (PGRs) play an essential role in determining the development pathway of plant cells and tissues in culture medium. The auxins, cytokinins and gibberellins are most commonly used plant growth regulators. The type and the concentration of hormones used depend mainly on the species of the plant, the tissue or organ cultured and the objective of the experiment (Ting, 1982). The high concentration of auxins generally favors root formation, whereas the high concentration of cytokinins promotes shoot regeneration. A balance of both auxin and cytokinin leads to the development of mass of undifferentiated cells known as callus.
The propagation from meristematic tissue generally provides a method of cleaning up material from viruses and other systemic pathogen infections. Micropropagation is a tissue culture (in vitro) method used for rapid and true to type multiplication of plants on artificial nutrient media under controlled environment and it is the most commercially exploited area of plant tissue culture, being widely used for production of quality planting material in vegetative propagated species. The most significant advantages offered by micropropagation are production of large of disease free propagules from a single plant in
a short period, propagation can be carried out throughout the year and the propagating material can be accommodated in a small space, reduction of labour costs for germplasm maintenance, avoidance of field inspections & environmental hazards, easy availability of material for micro propagation and rapid multiplication (Mtui, 2011). According to Chadha and Choudhary (2010) various prerequisite steps involved in production of pathogen free plantlets by meristem tip culture are: testing of parent material for the presence of viruses and similar pathogens (viroids and phytoplasmas), thermotherapy/chemotherapy of parent material if disease-free material is not available, excision of meristem tip under aseptic conditions, culture of apical dome plus one or two leaf primordia on suitable medium to produce plantlets, indexing of plantlets for presence or absence of viruses, plantlets transferred to soil, maintenance of pathogen free nuclear plant stocks and meristem culture is then followed by in vitro mass propagation of the virus-free plants thus obtained.
Technological Protocols
In recent past very fast developments have been taken placed in the area of plant cell culture-cum- microprogation with view of its viable commercial applications. For the purpose of micropropagation of planting materials the procedure of it starts with the selection of plant tissues (explant) from a disease free healthy, vigorous mother plant (Murashige, 1974). Any part of the plant (leaf, apical meristem, bud and root) can be used as explant. All the steps can be summarized into the following stages as shown in Figure 1 as documented by Hussain et at (2012).
-
-
- Stage 0: Preparation of donor plant- To enhance the probability of success, the mother plant should be ex vitro cultivated under optimal conditions to minimize contamination in the in vitro culture (Cassells and Doyle, 2005).
- Stage I: Initiation stage- In this stage an explant is surface sterilized and transferred into nutrient medium. Generally, the combined application of bactericide and fungicide products is suggested. The selection of products depends on the type of explant to be introduced. The surface sterilization of explant in chemical solutions is an important step to remove contaminants with minimal damage to plant cells (Hussain and Anis, 2009). The most commonly used disinfectants are sodium hypochlorite (Tilak et al, 2009), calcium hypochlorite (Garcia et al, 1999), ethanol (Singh and Gurung, 2009) and mercuric chloride (Hussain and Anis, 2009). The cultures are incubated in growth chamber either under light or dark conditions according to the
-
method of propagation.
-
-
- Stage II: Multiplication stage- This phase is to increase the number of propagules under which the number of propagules is multiplied by repeated subcultures until the desired number of plants is attained (Saini and Jaiwal, 2002).
- Stage III: Rooting stage- The rooting stage may occur simultaneously in the same culture media used for multiplication of the explants. However, in some cases it is necessary to change media, including nutritional modification and growth regulator composition to induce rooting and the development of strong root growth.
- Stage IV: Acclimatization stage- At this stage, the in vitro plants are weaned and hardened. Hardening is done gradually from high to low humidity and from low light intensity to high light intensity. The plants are then transferred to an appropriate substrate (sand, peat, compost etc.) and gradually hardened under greenhouse.
-
RECENT ADVANCES IN PLANT TISSUE CULTURE
Processes of somatic embryogenesis and organogenesis are the recent development in the area application of plant tissue culture by which plant regeneration takes place.
Somatic Embryogenesis
It is an in vitro method of plant regeneration widely used as an important biotechnological tool for sustained clonal propagation (Park et al, 1998). It is a process by which somatic cells or tissues develop into differentiated embryos. These somatic embryos can develop into whole plants without undergoing the process of sexual fertilization as done by zygotic embryos. The somatic embryogenesis can be initiated directly from the explants or indirectly by the establishment of mass of unorganized cells named callus (Suman and Kumar, 2016). Plant regeneration via somatic embryogenesis occurs by the induction of embryogenic cultures from zygotic seed, leaf or stem segment and further multiplication of embryos. Mature embryos are then cultured for germination and plantlet development, and finally transferred to soil. Somatic embryogenesis has been reported in many plants including trees and ornamental plants of different families. There are various factors that affect the induction and development of somatic embryos in cultured cells. A highly efficient protocol has been reported for somatic embryogenesis on grapevine (Jayasankar et al, 1999) that showed higher plant regeneration sufficiently when the tissues were cultured in liquid medium. Plant growth regulators play an important role in the regeneration and
proliferation of somatic embryos. Highest efficiency of embryonic callus was induced by culturing nodal stem segments of rose hybrids on medium supplemented with various PGRs alone or in combination (Xiangqian et al, 2002). The embryonic callus showed high germination rate of somatic embryos when grown on abscisic acid (ABA) alone. Somatic embryogenesis is not only a process of regenerating the plants for mass propagation but also regarded as a valuable tool for genetic manipulation. The process can also be used to develop the plants that are resistant to various kinds of stresses (Bouquet and Terregosa, 2003) and to introduce the genes by genetic transformation (Maynard et al, 2003). A successful protocol has been developed by using this tool for regeneration of cotton cultivars with resistance to Fusarium and Verticillium wilts (Han et al, 2009).
Organogenesis
It refers to the production of plant organs i.e. roots, shoots and leaves that may arise directly from the meristem or indirectly from the undifferentiated cell masses (callus). Plant regeneration via organogenesis involves the callus production and differentiation of adventitious meristems into organs by altering the concentration of plant growth hormones in nutrient medium. Skoog and Miller (1957) were the first who demonstrated that high ratio of cytokinin to auxin stimulated the formation of shoots in tobacco callus while high auxin to cytokinin ratio induced root regeneration.
- CRITICAL FACTORS INFLUENCING IN VITRO GROWTH
Choice of explant
The tissue which is obtained from the plant to culture is called an explant. Based on work with certain model systems, particularly tobacco, it has often been claimed that a totipotent explant can be grown from any part of the plant. In many species, explants of various organs vary in their rates of growth and regeneration, while some do not grow at all. The choice of explant material also determines if the plantlets developed via tissue culture are haploid or diploid. Also the risk of microbial contamination is increased with inappropriate explants. Thus it is very important that an appropriate choice of explant be made prior to tissue culture. The specific differences in the regeneration potential of different organs and explants have various explanations. The significant factors include differences in the stage of the cells in the cell cycle, the availability of or ability to transport endogenous growth regulators and the metabolic capabilities of the cells. The most commonly used tissue explants are the meristematic ends of the plants like the
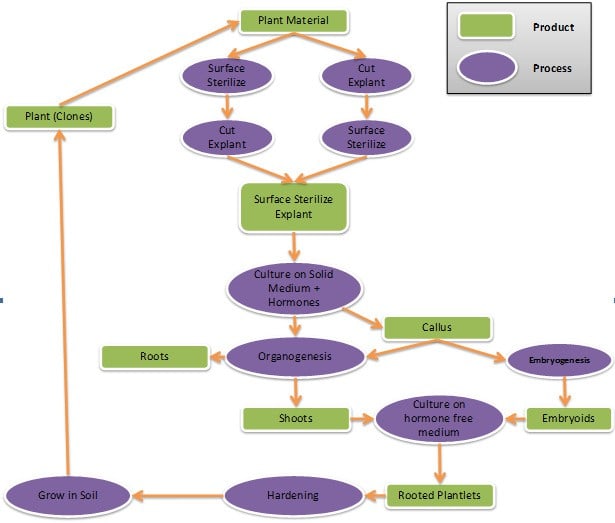
Fig. 1 : Step-wise summary of micropropagation via tissue culture.

Fig. 2 : Steps involved in hybrid plant production via protoplast fusion.
stem tip, auxiliary bud tip and root tip because these tissues have high rates of cell division and either concentrate or produce required growth regulating substances including auxins and cytokinins. Some explants, like the root tip, are hard to isolate and are contaminated with soil microflora that become problematic during the tissue culture process. Certain soil micro-flora can form tight associations with the root systems, or even grow within the root. Soil particles bound to roots are difficult to remove without injury to the roots, a circumstance that then allows microbial attack. These associated microfloras will generally overgrow the tissue culture medium before there is significant growth of plant tissue. Aerial (above soil) explants are also rich in undesirable microflora. However, they are more easily removed from the explant by gentle rinsing and the remainder usually can be killed by surface sterilization. Most of the surface microflora does not form tight associations with the plant tissue. Such associations can usually be found by visual inspection as a mosaic, de-colorization or localized necrosis on the surface of the explant. An alternative for obtaining uncontaminated explants is to take explants from seedlings which are aseptically grown from surface sterilized seeds. The hard surface of the seed is less permeable to penetration of harsh surface sterilizing agents, such as hypochlorite, so the acceptable conditions of sterilization used for seeds can be much more stringent than for vegetative tissues.
Explant Size and Thin Section Culture System
The induction of a desired morphogenic event in vegetative tissues by appropriate in vitro manipulations would probably be the most significant advancement in plant tissue culture. The success in achieving such directed morphogenic events are largely determined by the cultured tissue itself. Several explant-related factors appear to influence the organogenic potential of the cultured tissue (Benson, 2000). These include growth conditions, whole plant physiology and genotype of the source plant. In addition, a negative correlation between the explants size and the number of cells potentially available for organogenesis has also been recognized (Lakshmanan et al, 1995 & 1996, ). In an earlier study, Lakshmanan et al (1995) have shown that the production of orchid protocorms in vitro can be substantially improved by manipulating the size of the explant alone. For example, the number of protocorms produced by thin transverse sections (0.6 mm thick) derived from a single shoot tip (6-7 mm long) was 5 times greater than that produced by an intact shoot tip (6-7 mm long) cultured under identical conditions. A similar observation was also made recently in sugarcane. In this crop, leaf explants
produced numerous plants (> 50 per explant) when the thickness of the explant was reduced to 1 to 2 mm. This finding clearly indicates that the explant size plays a key role in the expression of organogenic potential of the cultured tissue. This explants size-based difference in organogenic capacity has since been successfully utilized to develop thin section culture system, a novel approach in plant regeneration
4.3 In vitro Environmental Conditions
Some interesting points of basic research that could improve our understanding and hence our ability to control in vitro plant regeneration and development remain under- explored such as by recirculating the liquid culture systems it will be feasible to monitor and continuously regulate the medium composition. We need to know more about the dynamics of mineral nutrition in vitro (Williams, 1995). Light quality is one of the potentially important environmental factors, as it has been shown to affect the direction of plant morphogenesis in vitro (Morini et al, 2000) and it also plays role in between gametophytic and sporophytic pathways. Tissue culture processes often involves extensive cutting and stress injury of tissues which may cause physiological changes in plants affecting the success response (Leon et al, 2001).
SCIENTIFIC-CUM-COMMERCIAL APPLICATIONS OF PLANT TISSUE CULTURE
TECHNOLOGY
Today plant tissue culture applications encompass much more than clonal propagation and micropropagation. The range of routine technologies has expanded to include somatic embryogenesis, somatic hybridization, virus elimination as well as the application of bioreactors to mass propagation and production of secondary metabolites. The applications of plant tissue culture may be categorized into two areas of common interest:
In Agricultural Science
Plant tissue culture is used widely in plant science with its commercial applications as- screening cells rather than plants for advantageous characters, e.g. herbicide resistance/ tolerance; large scale growth of plant cells in liquid culture inside bioreactors as a source of secondary products, like recombinant proteins used as biopharmaceuticals; to cross distantly related species by protoplast fusion and regeneration of the novel hybrid; embryo rescue (the resulting embryo as a result of cross pollination which would otherwise normally die is cultured in a medium to rescue it); for production of doubled monoploid plants from haploid cultures to achieve homozygous lines more rapidly in breeding programs, usually being done by treatment with colchicine which
causes doubling of the chromosome number; as a tissue for transformation, followed by either short term testing of genetic constructs or regeneration of transgenic plants; in vitro conservation of germplasm and so on. This technique is mainly used to conserve plant which do not produce seeds or which have recalcitrant seeds which cannot be stored under normal storage conditions in seed gene banks. Hence, vegetative propagated crops such as root and tubers, ornamentals, medicinal plants and many other tropical fruits have to be conserved by using tissue culture methods (Singh and Shetty, 2011).
Tissue culture has been introduced into agricultural practice at a rate without precedent which allows the production and propagation of genetically homogeneous, disease-free plant material by micropropagation methodology (Chatenet et al, 2001). Cell and tissue in vitro culture is a useful tool for the induction of somaclonal variation (Marino and Battistini, 1990). Genetic variability induced by tissue culture could be used as a source of variability to obtain new stable genotypes. Interventions of biotechnological approaches in in vitro regeneration, mass micropropagation techniques and gene transfer studies in tree species have been encouraging. In vitro cultures of mature and/or immature zygotic embryos are applied to recover plants obtained from inter- generic crosses that do not produce fertile seeds (Ahmadi et al, 2010). Genetic engineering can make possible a number of improved crop varieties with high yield potential and resistance against pests. Genetic transformation technology relies on the technical aspects of plant tissue culture and molecular biology for production of improved crop varieties, production of disease (virus)-free plants, genetic transformation, production of secondary metabolites, and production of varieties tolerant to salinity, drought and heat stresses and so on. Details of some prime applications of tissue culture in agricultural development are given as-
-
-
- Germplasm conservation : In vitro cell and organ culture offers an alternative source for the conservation of endangered genotypes (Sengar et al, 2010). Germplasm conservation worldwide is increasingly becoming an essential activity due to the high rate of disappearance of plant species and the increased need for safeguarding the floristic patrimony of the any country (Filho et al, 2005). Tissue culture protocols are of prime use in the situation for preservation of vegetative tissues when the targets for conservation are clones instead of seeds, to keep the genetic background of a crop and to avoid the loss of the conserved patrimony due to natural disasters, whether biotic or abiotic stress (Tyagi et al, 2007). The plant species which do not produce seeds
-
(sterile plants) or which have ‘recalcitrant’ seeds that cannot be stored for long period of time can successfully be preserved via in vitro techniques for the maintenance of gene banks. Cryopreservation plays a vital role in the long-term in vitro conservation of essential biological material and genetic resources. It involves the storage of in vitro cells or tissues in liquid nitrogen that results in cryo-injury on the exposure of tissues to physical and chemical stresses. Successful cryopreservation is often ascertained by cell and tissue survival and the ability to re-grow or regenerate into complete plants or form new colonies (Harding, 2004). It is desirable to assess the genetic integrity of recovered germplasm to determine whether it is ‘true-to-type’ following cryopreservation (Day, 2004). The fidelity of recovered plants can be assessed at phenotypic, histological, cytological, biochemical and molecular levels, although, there are advantages and limitations of the various approaches used to assess genetic stability (Harding et al, 2005; Suman et al, 2015). Cryobionomics is a new approach to study genetic stability in the cryopreserved plant materials Harding, 2010). The embryonic tissues produced by tissue culture are cryopreserved for future use or for germplasm conservation (Corredoira et al, 2004).
-
-
- Embryo culture: It is a type of plant tissue culture that is used to grow embryos from seeds and ovules in a nutrient medium. In embryo culture, the plant develops directly from the embryo or indirectly through the formation of callus and then subsequent formation of shoots and roots. The technique has been developed to break seed dormancy, test the vitality of seeds, production of rare species and haploid plants (Holeman, 2009). It is an effective technique that is employed to shorten the breeding cycle of plants by growing excised embryos and results in the reduction of long dormancy period of seeds. Intra-varietal hybrids of an economically important energy plant “Jatropha” have been produced successfully with the specific objective of mass multiplication (Mohan et al, 2011). Somatic embryogenesis and plant regeneration has been carried out in embryo cultures of Jucara Palm for rapid cloning and improvement of selected individuals (Guerra and Handro, 1988). In addition, conservation of endangered species can also be attained by practicing embryo culture technique. A successful protocol has been developed for the in vitro propagation of Khaya grandifoliola, a plant of high economic value for timber wood and for medicinal purposes as well, by excising embryos culture from mature seeds (Okere and Adegey, 2011). Plant tissue culture technology has an important application in forestry by offering a mean of propagation of elite individuals where the selection and improvement
-
Table 1 : Some of secondary metabolites produced in plant cell suspension culture and being used in pharmaceutical industries.
|
Secondary metabolite |
Plant name |
Reference |
|
Vasine |
Adhatoda vasica |
Shalaka and Sandhya (2009) |
|
Artemisinin |
Artemisia annua |
Baldi and Dixit (2008) |
|
Azadirachtin |
Azadirachta indica |
Sujanya et al (2008) |
|
Cathin |
Brucea javanica |
Wagiah et al (2008) |
|
Capsiacin |
Capsicum annum |
Umamaheswai and Lalitha (2007) |
|
Sennosides |
Cassia senna |
Shrivastava et al (2006) |
|
Ajmalicine |
Catharanthus roseus |
Zhao et al (2001) |
|
Secologanin |
Contin et al (1999), |
|
|
Indole alkaloids |
Moreno et al (1993) |
|
|
Vincristine |
Lee-Parsons and Rogce (2006) |
|
|
Stilbenes |
Cayratia trifoliata |
Roat and Ramawat (2009) |
|
Berberin |
Coscinium fenustratum |
Khan et al (2008) |
|
Sterols |
Hyssopus officinalis |
Skrzypek and Wysokinsku (2003) |
|
Shikonin |
Lithospermum erythrorhizon |
Tabata and Fujita (1985) |
|
Ginseng saponin |
Panax notoginseng |
Zhong et al (1999) |
|
Podophyllotoxin |
Podophyllum hexandrum |
Chattopadhyay et al (2002) |
|
Taxane Paclitaxel |
Taxus chinensis |
Wang et al (1999) |
of natural population is not feasible and viable too.
-
-
- Genetic transformation : Genetic transformation is the most important aspect of plant cell- tissue culture that provides the mean of transfer of genes with desirable trait into host plants with ultimate recovery of transgenic plants (Hinchee et al, 1994). It has a great potential of genetic improvement of various crop plants by integrating with plant biotechnology and breeding programmes. It has a prioritized promising role for the introduction of agronomically important traits such as increased yield, better quality and enhanced resistance to pests, diseases and abiotic stresses (Sinclair et al, 2004; Sharma et al, 2010; Kumar et al, 2016). Genetic transformation in plants can be achieved by either vector- mediated (indirect gene transfer) or vector less (direct gene transfer) method (Sasson, 1993). Among vector dependant gene transfer methods, Agrobacterium– mediated genetic transformation is most widely used for the expression of foreign genes in plant cells. Successful introduction of agronomic traits in plants was achieved by using root explants for the genetic transformation (Franklin and Lakshmi, 2003). Regeneration of disease or viral resistant plants is now achieved by employing genetic transformation technique. Successfully transgenic plants of potato resistant to potato virus Y (PVY) has been developed thus resolving a major threat to potato crop worldwide (Bukovinszki et al, 2007).
- Protoplast fusion : Somatic hybridization being achieved by protoplasm fusion is an important tool of plant breeding and crop improvement by the production of inter-specific and inter-generic hybrids. It involves the fusion of protoplasts of two different genomes followed by the selection of desired somatic hybrid cells and regeneration of hybrid plants (Evans and Bravo, 1988). Practically in the crop improvement programmes protoplast fusion is efficiently used as a mean of gene transfer with desired trait from one species to another (Brown and Thorpe, 1995). Somatic hybrids were produced by fusion of protoplasts from rice and ditch reed using electrofusion treatment for salt tolerance (Mostageer and Elshihy, 2003).
-
In the recent years, in vitro protoplast fusion tool has opened a way of developing unique hybrid plants by overcoming the barriers of sexual incompatibility. It has been applicable in horticultural industry to create new hybrids with increased fruit yield and better resistance to diseases. Successful viable hybrid plants were obtained when protoplasts from citrus were fused with other related Citrinae species (Motomura et al, 1997). The potential of somatic hybridization in important crop plants is best illustrated by the production of intergeneric hybrid plants among the members of Brassicaceae (Toriyama, 1987). In wheat crop for the purpose of gene pool recovery and improvement by resolving the problem of loss of chromosomes and decreased regeneration capacity,

Fig. 3 : Shoot tip culture for banana micropropagation: a. sword sucker and explant; b. shooting after apical disabling; c. proliferation; d. multiple shooting; e. rooting; f. nursery hardening (Source: Singh et al (2011).
successful protocol of protoplasm fusion has been established and used for the production of somatic hybrid plants by using two types of wheat protoplast as recipient and protoplast of Haynaldia villosa as a fusion donor (Liu et al, 1988). Steps involved in protoplast fusion are represented by figure 2.
-
-
- Haploid production: By use of the tissue culture techniques it is possible to produce homozygous plants in relatively short time period through the protoplast, anther and microspore cultures instead of conventional breeding (Morrison and Evans, 1998). Haploids are sterile plants having single set of chromosomes which are converted into homozygous diploids by spontaneous or induced chromosome doubling. The doubling of chromosomes restores the fertility of plants resulting in production of double haploids with potential to become pure breeding new cultivars (Basu et al, 2011). The term
-
androgenesis refers to the production of haploid plants from young pollen cells without undergoing fertilization. Sudherson et al. (2008) reported haploid plant production of sturt’s desert pea by using pollen grains as primary explants via tissue culture. Now a day the haploidy technology has become an integral part of crop improvement programmes through plant breeding by speeding up the production of inbred lines (Bajaj, 1990) and overcoming the constraints of seed dormancy and embryo non-viability (Yeung et al, 1981). The technique has a remarkable use in genetic transformation by the production of haploid plants with induced resistance to various biotic and abiotic stresses. Introduction of genes with desired trait at haploid state followed by chromosome doubling led to the production of double haploids inbred wheat and drought tolerant plants were attained successfully (Chauhan and Khurana, 2011).
Table 2 : Secondary metabolites of commercial importance produced in plant hairy root culture and being used in pharmaceutical industries.
|
Secondary metabolite |
Plant name |
Reference |
|
Rosmarinic acid |
Agastache rugosa |
Lee et al (2007) |
|
Deoursin |
Angelica gigas |
Xu et al (2008) |
|
Resveratol |
Arachys hypogaea |
Kim et al (2008) |
|
Tropane |
Brugmansia candida |
Marconi et al (2008) |
|
Asiaticoside |
Centella asiatica |
Kim et al (2007) |
|
Rutin |
Fagopyrum esculentum |
Lee et al (2007) |
|
Glucoside |
Gentiana macrophylla |
Tiwari et al (2007) |
|
Glycyrrhizin |
Glycyrrhiza glabra |
Mehrotra et al (2008) |
|
Shikonin |
Lithospermum erythrorhizon |
Fukui et al (1998) |
|
Glycoside |
Panax ginseng |
Jeong and Park (2007) |
|
Plumbagin |
Plumbago zeylanica |
Verma et al (2002) |
|
Anthraquinone |
Rubia akane |
Park and Lee (2009) |
|
Silymarin |
Silybium marianum |
Rahnama et al (2008) |
|
Flavonolignan |
Silybium mariyanm |
Alikaridis et al (2000) |
|
Vincamine |
Vinca major |
Tanaka et al (2004) |
|
Withanoloid A |
Withania somnifera |
Murthy et al (2008) |
In Pharmaceutical Science
standard phytochemicals in large volumes but also eliminate the presence of interfering compounds that occur in the field-grown plants (Lila, 2005). The major advantage of the cell cultures include synthesis of bioactive secondary metabolites, running in controlled environment, independently from climate and soil conditions (Karuppusamy, 2009). For the industrial production point of view, a number of different types of bioreactors have been in use for mass cultivation of plant cells. The first commercial application of large scale cultivation of plant cells was carried out in stirred tank reactors of 200 liter and 750 liter capacities to produce shikonin by cell culture of Lithospermum erythrorhizo (Payne et al, 1987). A number of medicinally important alkaloids, anticancer drugs, recombinant proteins and food additives are produced in various cultures of plant cell and tissues in bioreactors. Advances in the area of cell cultures for the production of medicinal compounds has made possible the production of a wide variety of pharmaceuticals like alkaloids, terpenoids, steroids, saponins, phenolics, flavanoids and amino acids
In the era of ever demanding development of pharmaceutical industry, plant cell tissue culture holds great promise for controlled production of myriad of useful secondary metabolites (Vijayasree et al, 2010). Plant cell cultures have provided an efficient platform for the production of valuable therapeutic secondary metabolites by utilizing the merits of whole-plant systems with those of microbial and animal cell cultures (Hellwig et al, 2004). In the want for alternatives to production of medicinal compounds from plants, biotechnological approaches, specifically plant tissue cultures, are found to have potential as a supplement to traditional agriculture in the industrial production of bioactive plant metabolites (Rao and Ravishankar, 2002). During the last decade exploration of the biosynthetic capabilities of various cell cultures has been carried out by a group of plant scientists and microbiologists in several countries (Siahsar et al, 2011) particularly adopting either of one of following culture modules.
-
-
- Cell suspension culture : Cell suspension culture systems are used now days for large scale culturing of plant cells from which secondary metabolites are extracted. A suspension culture is developed by transferring the relatively friable portion of the callus into liquid medium and is maintained under suitable conditions of aeration, agitation, light, temperature and other physical parameters (Chattopadhyay et al, 2002; Rao and Ravishankar, 2002). Cell cultures cannot only yield defined
-
(Vijayasree et al, 2010; Yesil-Celiktas et al, 2010).
Advances in scale up approaches and immobilization techniques contribute to a considerable increase in the number of applications of plant cell cultures for the production of compounds with a high added value. Some of the secondary plant products obtained from cell suspension culture of various plants are given in Table 1 (Source: Hussain et al, 2012).
-
-
- Hairy root cultures : This system based on inoculation with Agrobacterium rhizogenes has become popular in the last two decades as a method of producing secondary metabolites synthesized in plant roots (Palazon et al, 1997). Organized root cultures can make a significant contribution in the production of secondary metabolites. Most of the research efforts that use differentiated cultures instead of cell suspension cultures have focused on transformed (hairy) roots. Agrobacterium rhizogenes causes hairy root disease in plants. The neoplastic (cancerous) roots produced by A. rhizogenes infection are characterized by high growth rate, genetic stability and growth in hormone free media (Hu and Du, 2006). High stability (Giri and Narasu, 2000) and productivity features allow the exploitation of hairy roots as valuable biotechnological tool for the production of plant secondary metabolites (Pistelli et al, 2010). These genetically transformed root cultures can produce high level of secondary metabolites comparable to that of intact plants (Srivastava and Srivastava, 2007). Nutrient’s composition optimizing in hairy root cultures is the critical
-
point to gain a high production of secondary metabolites (Hu and Du, 2006). Some of the secondary plant products obtained from hairy root culture of various plants are shown in Table 2 (Source: Hussain et al, 2012).
Innovative Applications
The greatest value of the plant cell/tissue culture lies not so much in their application to mass clonal propagation (micropropagation) only but rather in its role underpinning development and application in plant improvement, molecular biology and bio-processing, as well as, its importance in research. The applications of it go well beyond the any bound in agriculture and other allied fields of food productions as follows-
-
-
- As bioreactors : It is a well-established fact that most plants in culture grow better in liquid than on solid media. To further enhance the productivity of liquid culture systems, several innovative approaches were adapted depending on the final product desired and the species investigated (Aitken-Christie et al, 1995). Bioreactors for plant culture are the most prominent being adapted for a number of species. Since Murashige (1974) introduced the basic micropropagation plan, the application of bioreactors is one of the major developments that have occurred in the plant tissue culture industry. Compared to traditional tissue culture techniques, bioreactor systems offer several advantages; they are time and labour-saving, relatively easy to scale-up, allow enhanced growth and multiplication (e.g. by forced aeration) and improved nutrient availability due to the use of liquid medium. Several new strategies have been adapted to develop bioreactors suitable for various plant species and their specific requirements (Aitken-Christie et al., 1995; Paek et al, 2001). As per Lee (2004) the principal systems are-
-
- Aeration-agitation bioreactor
- Spin filter bioreactor
- Gaseous phase bioreactor
- Rotating drum bioreactor
- Air-driven bioreactor
These basic systems have already been used for the mass production of over 80 crops (Takayama, 1991) and are now being evaluated for production of several other plant species (Paek et al, 2001). As a plant production technique, bioreactors are far superior to traditional in vitro methods for all the species thus far tested. It is worth noting that with bioreactors, even the difficult-to- propagate woody and tree species can be produced relatively easily at high frequency. For instance, an efficient, somatic embryo-based mass propagation system
for the recalcitrant species Coffea arabica was recently developed using a bioreactor (Etienne-Barry et al, 1999). The normal and uniform development of coffee embryos achieved with the use of a bioreactor allowed direct sowing of embryos in the field, resulting in rapid crop establishment. In brief, bioreactors have the potential to improve product quality and substantially reduce the cost of micropropagation, but further development of technology is required to realize any commercial benefit from this system.
-
-
- As in vitro mycorrhization : Traditionally, aseptic conditions were considered essential for plant tissue culture systems. But now, attention has turned to the possible beneficial effects of microorganisms in in vitro plant cultures. For example, the root endophyte Piriformospora indica promotes explants hardening (Sahay and Varma, 1999), Psuedomonas spp. can reduce hyperhydricity (Bela et al, 1998) and Bacillus pumilus, Alcaligenes faecalis and Psuedomonas spp. improve shoot multiplication (Monier et al, 1998). Mycorrhization in micropropagation, particularly the use of arbuscular mycorrhizal fungi (AMF), is now gaining momentum due to its demonstrated positive impact on post-transplant performance of in vitro grown plants (Lovato et al, 1996; Rai, 2001). Improved nutrient uptake, water relations, aeration, soil pH balance (Sylvia, 1998; Bisht et al, 2009) and their potential use as bioregulators (Lovato et al, 1996) have recently exemplified the research interest in AMF, contributing to the development of effective AMF production methods, mycorrhization of in vitro plants and screening for efficient AMF strains. The potential of different AMFs for application in commercial micro- propagation industries can now be tested using an array of tools (Srivastava et al, 2011).
-
BANANA AS A PRIME-POTENTIAL HORTICULTURAL CROP AND ITS
STANDARDIZED MICROPROPAGATION PROTOCOLS
Banana evolved in the humid tropical regions of S.E. Asia with India as one of its centres of origin. Modern edible varieties have evolved from the two species – Musa acuminata and Musa balbisiana and their natural hybrids, originally found in the rain forests of S.E. Asia. During the seventh century AD its cultivation spread to Egypt and Africa. At present banana is being cultivated throughout the warm tropical regions of the world between 300 N and 300 S of the equator. Banana and Plantains (Musa spp.) are some of the earliest crop plants having been domesticated by humans. Bananas are consumed as ripe fruit, whereas plantains, which remain starchy even when fully ripe, need cooking for palatability and
Table 3 : Commercial cultivars of banana popularly propagated in India.
|
State |
Varieties grown |
|
Andhra Pradesh |
Dwarf Cavendish, Robusta, Rasthali, Amritpant, Thellachakrakeli, Karpoora Poovan, Chakrakeli, Monthan and Yenagu Bontha |
|
Assam |
Jahaji (Dwarf Cavendish), Chini Champa, Malbhog, Borjahaji (Robusta), Honda, Manjahaji, Chinia (Manohar), Kanchkol, Bhimkol, Jatikol, Digjowa, Kulpait, Bharat Moni |
|
Bihar |
Dwarf Cavendish, Alpon, Chinia , Chini Champa, Malbhig, Muthia, Kothia , Gauria |
|
Gujarat |
Dwarf Cavendish, Lacatan, Harichal (Lokhandi), Gandevi Selection, Basrai, Robusta, G-9, Harichal, Shrimati |
|
Jharkhand |
Basrai, Singapuri |
|
Karnataka |
Dwarf Cavendish, Robusta, Rasthali, Poovan, Monthan, Elakkibale |
|
Kerala |
Nendran (Plantain), Palayankodan (Poovan), Rasthali, Monthan, Red Banana, Robusta |
|
Madhya Pradesh |
Basrai |
|
Maharashtra |
Dwarf Cavendish, Basrai, Robusta, Lal Velchi, Safed Velchi, Rajeli Nendran, Grand Naine, Shreemanti, Red Banana |
|
Orissa |
Dwarf Cavendish, Robusta, Champa, Patkapura (Rasthali) |
|
Tamil Nadu |
Virupakshi, Robusta, Rad Banana, Poovan, Rasthali, Nendran, Monthan, Karpuravalli, Sakkai, Peyan, Matti |
|
West Bengal |
Champa, Mortman , Dwarf Cavendish, Giant Governor, Kanthali, Singapuri |
consumption. Originally crops from humid tropics, they have acclimatized to a broad range of climatic conditions. While bananas have come to occupy the status of a high value, commercial crop, plantains have remained a staple food of many ethnic groups. Irrespective of their commercial status, banana and plantains are referred as ‘Poor man’s apple’.
Banana is globally ranked fourth, next to rice, wheat and maize in terms of gross value of production. It is a major staple food crop for millions of people as well as provides income through local and international trade. Among the starchy staple food crops, banana ranks third with respect to the total production. Though cassava and sweet potato are positioned as first and second, banana and plantain have almost equal importance in all the tropical regions of the world. Traditional bananas and other species of family Musaceae have been the major calorie source of many ethnic tribes of Africa and Pacific Islands. High consumption of bananas has been reported in small countries of Pacific Islands like Samoa (132 Kcal) and Vanuatu (92 Kcal). Bananas also find importance in the diet of Caribbean (Haiti and Dominican Republic) and Latin American countries like Ecuador and Brazil (Singh et al, 2011). Banana (Musa sp.) is the second most important fruit crop in India next to mango. Its year round availability, affordability, varietal range, taste, nutritive and medicinal value makes it the favorite fruit among all classes of people. It has also good export potential.
Commercial Cultivars
Commercially, bananas are classified as dessert types and culinary types. The culinary types have starchy fruits and are used in the mature unripe form as vegetables.
Important cultivars include Dwarf Cavendish, Champa, Malbhog, Robusta, Monthan, Poovan, Nendran, Red banana, Nyali, Safed Velchi, Basrai, Ardhapuri, Rasthali, Karpurvalli, Karthali and Grand Naine etc. Among all these cultivars, Grand Naine, an imported variety from Israel is gaining popularity and may soon become the most preferred variety due to its tolerance to abiotic stresses and good quality bunches. Fruit develops attractive uniform yellow colour with better shelf life & quality than other cultivars. Important banana varieties cultivated in different states of India are given in table 3.
Micropropagation Protocols
Traditionally, banana is grown as a perennial crop where the plant is allowed to produce continuous shoots from a subterranean stem. But, the yields fall after three to five years and decline rapidly after ten to fifteen years. The need to shift to cyclic replacement with a new plantation comprising cycles of one crop and one ratoon has been realized only recently in most Asian countries. Natural calamities such as typhoons, floods, droughts and occasional volcanic eruptions cause devastating losses in banana production. The consequent need for fresh seedlings at regular intervals has led to very large increase in the demand for clean planting material. Banana is vulnerable to a number of biotic and abiotic stresses which limit its production, particularly among small and marginal farmers with limited resources. BBTV (Banana bunchy top virus), CMV (Cucumber mosaic virus), BSV (Banana streak virus) and BBMV (Banana bract mosaic virus) are the four most important virus diseases affecting bananas. In addition, banana is an attractive host for nematodes, particularly Pratylenchus coffeae,
Meloidogyne incognita, Helicotylenchus multicinctus and Radopholus similis. The pests also spread through transportation of non-quarantined planting material. Insect pests like, banana weevils (Odoiporus longicollis, and Cosmpolites sordidus) which till recently had a limited presence in some states of India have now spread to Bangladesh and beyond. Disease and pest pressure on Asian bananas is unlikely to lessen in the foreseeable future (Singh et al, 2011). Tissue culture technology has been the foundation of high quality, disease free planting material production of banana at a mass scale.
Banana is a crop with dual propagation abilities, sexual through seeds and asexual through suckers. Seed propagation is common in wild species which are diploid and undergo normal meiosis, fertilization and seed set. All cultivated commercial bananas are triploid and sterile, excepting a few parthenocarpic AA and AB diploids. Sucker propagation is the only natural means of their perpetuation; artificial methods of propagation include macropropagation and micropropagation: most commonly by using shoot tips. Micropropagation is the practice of rapidly multiplying stock plant material to produce a large number of progeny plants under aseptic conditions using modern plant tissue culture methods. A common protocol by use of shoot tip as explant has been well documented by Singh et al (2011) and given below-
The earliest reports of in vitro culture of bananas came from Taiwan in the 70’s (Ma and Shii, 1974; Ma et al, 1978). Most commonly shoot tips can be extracted from the pseudostem, suckers, peepers, lateral buds or even small eyes which contain a shoot meristem (Jarret et al, 1985; Vuylsteke and De Langhe, 1985). Although all of them behave similarly under in vitro conditions, peepers and sword suckers are preferred because of their ease of handling and the minimum damage caused to the parent stool during their removal. It is always better to collect the explants from flowering plants so as to ascertain their trueness to type.
The critical steps followed for production of micropropagation based banana planting material are:
- Selection of quality explant material
- Culture medium selection
- Culture initiation,
- Culture proliferation,
- Rooting and primary hardening accompanied by rouging,
- Secondary hardening accompanied by rouging,
- Manuring and plant protection in nursery
- Field planting and initial management
- Fidelity testing and virus indexing at various stages of mass multiplication.
-
- Selection of quality explant materials: Choice of explant is vital for which purpose well maintained mother plants should be selected. Sword suckers should be healthy and not less than 60-80 days of age while the growing meristem should be of 1.0 cm3 in size.
- Culture medium selection: Success of in vitro culture depends largely on the choice of nutrient medium, including its chemical composition and physical form (Murashige, 1974). Several media formulations have been reported for banana shoot tip culture but nearly half of them are modified MS media (Brown et al, 1995). Other popular media include B5 (Gamborg et al, 1968),
-
SH (Schenk and Hildebrant, 1972), N6 (Chu et al, 1975),
and LS (Linsmaier and Skoog, 1975) media. The culture media vary in both type and concentration of the components, but all have similar basic components of growth regulators, nitrogen, carbohydrates, inorganic macro and micronutrients, vitamins and organic additives (table 4). Generally, the cultures are established on a separate initiation medium, which has a lower concentration of cytokinin than the multiplication medium to which the cultures are subsequently transferred (Jarret et al, 1985; Novak et al, 1989).
After autoclave sterilization, the culture medium is stored in a clean dust free chamber for 1-2 days before use in order to check for any contamination. Bacterial contamination may be observed, particularly during the rainy season. Use of Cefotaxime in the initiation and subsequent subcultures helps to overcome even latent bacterial contaminations.
-
-
- Culture initiation : The sword suckers of 2- 3 months are removed from healthy disease free mother plants for shoot tip culture as given in figure 3a. The suckers are cut to expose the shoot tip of 10 cm3 and cut further to about 3 cm diameter and 5 cm length. The explant should be carefully cut to avoid injury to the growing meristem. The shoot tips are washed in tap water and transferred to a container with 0.1% mercuric chloride for 10 min and then to 0.1% cetrimide. Then the shoot tips are washed thoroughly under running tap water to remove all traces of the chemicals. Using sharp sterile blade, one or two outer juvenile leaves and the corm base are trimmed out. Afterwards, the shoot tips are washed three times in sterile water in aseptic condition (under laminar air flow) disinfected with 5% sodium hypochlorite and later with 0.1% mercuric chloride each for 15 minutes. To avoid bacterial contamination, use of
-
Table 4 : The composition of initiation, multiplication and rooting media used at the National Research Centre for Banana, India (NRCB). Source: Singh et al (2011)
|
Culture media used at different stages of banana micropropagation (for one litre) |
|||
|
Ingredients |
Initiation medium |
Shoot multiplication medium |
Rooting medium |
|
Activated charcoal |
– |
– |
2.50 mg |
|
Agar |
– |
7.5 g |
7.5 g |
|
Ammonium nitrate |
1.65 g |
1.65 g |
1.65 g |
|
L-ascorbic acid |
10.0 mg |
10.0 mg |
– |
|
6-bezyl amino purine |
4.0 mg |
4.0 mg |
– |
|
Boric acid |
6.2 mg |
6.2 mg |
6.2 |
|
Calcium chloride |
440.0 mg |
440.0 |
440.0 |
|
Cobalt chloride |
0.025 mg |
0.025 |
0.025 |
|
Copper sulphate |
0.025 mg |
0.025 |
0.025 |
|
Ferric EDTA |
36.7 mg |
36.7 |
36.7 |
|
Glycine |
2.0 mg |
2.0 mg |
2.0 mg |
|
Indole-3-acatic acid |
1.0 mg |
1.0 mg |
– |
|
Indole-3-butyric acid |
– |
– |
1.0 mg |
|
Magnese sulphate |
22.3 mg |
22.3 |
22.3 |
|
Magnesium sulphate |
370.0 mg |
370.0 |
370.0 |
|
Myo-inositol |
100.0 mg |
100.0 mg |
100.0 mg |
|
-Naphaleneacetic acid |
– |
– |
2.0 mg |
|
Nicotinic acid |
0.5 mg |
0.5 mg |
0.5 mg |
|
Potassium dihydrogen ortho phosphate |
170.0 mg |
170.0 |
170.0 |
|
Phytal gel |
2.0 g |
– |
– |
|
Potassium iodide |
0.83 mg |
0.83 |
0.83 |
|
Potassium nitrate |
1.9 g |
1.9 g |
1.9 g |
|
Pyridoxine hydro chloride |
0.5 mg |
0.5 mg |
0.5 mg |
|
Sodium molybdate |
0.25 mg |
0.25 mg |
0.25 mg |
|
Sucrose |
30.0 g |
30.0 g |
30.0 g |
|
Thiamine hydrochloride |
0.1 mg |
0.1 mg |
0.1 mg |
|
Zinc sulphate |
8.6 mg |
8.6 |
8.6 |
Cefotaxime (0.1%) in the initiation medium is also advised.
Surface sterilized shoot tips are washed three times using sterile water. The outer surface of explant exposed to sterilizing agent is removed and the explants trimmed using surgical blade (No. 22) to bring the final size to about 3-4 cm length and 1-2 cm diameter (Fig. 3a). The explants are inoculated under sterile conditions in 30 ml of initiation medium in a 250 ml glass jar container. pH is
usually maintained at 5.8, which is prone to changes over culture duration. The optimum incubation temperature should be in the range of 24-26°C. Generally the light intensity is maintained at 1,500-3,000 lux. Higher levels of 3,000-10,000 lux during later stages improve the survival rate of plantlets upon transfer to soil. Initially, the cultures are maintained at 16 h light/8 h dark cycle and once after rooting they are shifted 14 h light/10 h dark cycle.
Decapitation and wounding of shoot tips are carried out to overcome apical dominance and to encourage axillary bud proliferation. But injuring the apical bud through transverse sections, either four or eight cuts, is a much preferred method. Injuring the explant encourages more production of phenols, but it can be kept at minimum using antioxidants like ascorbic acid.
-
-
- Culture proliferation: First subculture is done after 20-25 days of initiation when the explants turn green in colour. The cultures are first checked for contamination, in general symptoms of fungal contamination appear within one week and bacterial contamination symptoms like change of medium colour and texture or visible colonies appear within one week to one month. For subculturing, the outer dead tissue from the base of explant is removed and one or two leaf bases are peeled till the fresh meristematic tip gets exposed. The apical meristem is cut with two gentle cross incisions and the explant is transferred to subculture medium. During 20-25 days after the first subculture, the central meristem produces clusters of proliferating buds and one to three axillary buds get regenerated from the basal parts of explants around the central apical meristem (Fig. 3b). The number of axiliary buds developed during first and second subculture range from 1 to 5 depending on genomic constitution of the variety. In general, diploids like Matti, Anaikomban and Senna Chenkadali produce more buds than commercial cultivars. Among the latter, the number of buds produced during subculture is high in Cavendish (Robusta, Grand Naine – AAA genome) group followed by Plantain (Nendran – AAB genome) and Monthan (ABB genome) types.
-
Subsequent subculture is done by trimming the tip of emerging axillary buds and removal of dead tissue at the base of explant by gentle scratching. Clusters of proliferating buds develop during third and fourth subculture (Fig. 3c). For further subculturing, the explant is cut into three to four pieces and each slice with two to three proliferating clusters is inoculated to individual culture bottles. This subculture cycle is repeated at 3-4 weeks interval to increase the proliferation rate. During fourth and fifth subcultures, a single clump contains about
15-25 proliferating shoots. After 5-6 subculture cycles, the proliferated buds (Fig. 3d) are transferred to rooting medium containing IBA and activated charcoal. After a month, the rooted plantlets are ready for hardening (Fig. 3e). To minimize somatic variation, the subculturing is restricted to a maximum of seven cycles when each bottle contains 25-30 plantlets with well developed shoots and roots. Experimentally it has been demonstrated that proliferating shoots can be transferred to polybags (10- 20 cm size) having rooting media under green house. This reduces cost and enhances better establishment. Polybag provides enough space for plant growth and natural light enhances the process of hardening.
-
-
- Rooting and primary hardening accompanied by rouging : Once the plantlets are ready for shifting outside the laboratory, they are carefully acclimatized to adapt to the green house and later to least protected field conditions (Fig. 3f). During hardening, the plantlets undergo physiological adaptation to changing external factors like water, temperature, relative humidity and nutrient supply.
-
The plantlets from culture vessels/bottles are moved from the laboratory to a room at ambient temperature and kept open for 4-6 days. Later they are shifted to green house for primary hardening where they are first gently washed free of agar medium. This is important as sucrose in agar encourages microorganisms. 8 cm shoots with 3-4 ramified roots are planted in individual micropots in a protray. In places where weather is conducive (24- 26°C temperature and more than 80% humidity), the plantlets are hardened for 4-6 weeks in mini-sand beds. During this period, 90-95% humidity is maintained for the initial 6-8 days under diffused light. The humidity is slowly reduced to 70%, light intensity raised to normal and temperatures brought to 26°C by the end of 6 weeks.
Structures used for primary hardening vary with the climatic conditions. These can be highly sophisticated with UV-stabilized polysheet covering, multiple misting options, thermal shade net and auto-monitoring of light intensity, temperature and humidity. On the other hand, the structures can be simple with polycarbonate roofing, shade net on all sides with fogger facilities. Temperature, RH and light intensities are monitored manually using thermometer, hygrometer and lux meter, respectively.
Planting media for primary hardening range from sieved sand augmented with nutrition to mixtures of cocopeat and Soilrite with fine sand in equal proportions. NPK is provided in liquid form on weekly basis.
-
-
- Secondary hardening accompanied by rouging: After primary hardening for 5-6 weeks, the
-
plantlets are transferred from micropots to polybags. Base substrate is generally soil and sand along with low cost materials like coir pith, sawdust or rice husk. Organic manure is either in the form of farm yard manure or poultry manure. Even pressed mud, a by-product of sugar factories, has been found to provide best substrate for secondary hardening along with soil (Vasane et al, 2006). Plantlets from micropots are, dipped in fungicide solution (0.1% bavistin) and planted in polybags containing suitable substrate. Initially, these are maintained in low light intensity shade nets and 70% RH. The plants are hardened by gradually increasing the light intensity and reducing RH (40%). After 5-6 weeks, the plants become ready for field planting having 3-5 well developed leaves and a good mass of fibrous roots. During both primary and secondary hardening, the stocks should be rouged for variants at weekly intervals. These could include vegetative deformities like dwarfism, leaf variegation, and rosette foliage and leaf crinkiness. Other precautions to be followed are:
- The rooting media should be completely free from pathogens.
- Water used for irrigating the plants should be free from pests and pathogens.
- Sample plants from each batch should be randomly virus indexed (at least 10 plants from each batch/ explant)
- While shifting primary hardened plantlets, two longitudinal cuts should be given to the micropots to facilitate further corm growth.
-
- Manuring and plant protection in nursery: Plantlets should be 2-3 weeks old before any fertilizer is applied. 100 ml water containing 0.5 g urea, 2 g superphosphate and 1 g muriate of potash can be applied per plant. The manuring is repeated by doubling the dosage after three weeks. Spraying of commercially available micronutrient mixtures during sixth week helps in better establishment both in nursery and field. Strict sanitary measures are adopted in the nursery to avoid the risk of damage by pests and diseases either through substrate or irrigation water.
- Field planting and initial management: 20- 30 cm tall plants with 3-5 broad leaves are ready for field planting (Figs. 4 & 5). At the time of planting, 10 g of Carbofuron is applied per plant. Watering is done soon after field planting as young micropropagated plants are sensitive to dry weather and heat. Since these are also highly susceptible to bacterial rot (Erwinia rot), within 3 days of planting the soil around the plants is drenched with 500 ml of 0.1 % Emisson (methyl ethoxy mercuric
-
chloride). Recommended package of practices is strictly followed to achieve successful field establishment and subsequent vigorous growth (Figs. 4 & 5 & Flow chart Fig. 6).
-
-
- Testing for genetic fidelity and virus infection: Virus indexing and genetic fidelity testing are important to produce good quality disease free planting material using tissue culture technology by adopting standardize protocols.
-
Shoot tip cultures preserve genetic stability much better than callus or cell suspension cultures, yet somaclonal variation seems to be widespread among plants regenerated from banana shoot tip cultures. Commercial varieties like Robusta, Grand Naine, Dwarf Cavendish, Shrimanthi and Madhukar are highly susceptible to these variations and the off-types number up to 74%. High level of variation is not desirable as it defeats the purpose of clonal reproduction and majority of the off-types are agronomically inferior to the parental clone. Banana and plantains have a flexible genetic make and its genetic stability under cultive is strongly influenced by external factors like growth regulators, duration of culture etc. Mild stress under in vitro results in reverting of the clone to its parental type. For example, Robusta, a Cavendish clone frequently reverts to its original type Dwarf Cavendish which is not acceptable for its low stature and poor yield. Hence, genetic fidelity testing using preferably molecular marker techniques is essential to ensure the supply of true to type quality planting material. At NRCB, besides phenotype PCR technique with ISSR markers is being successfully used for screening off-types in banana as in table 5. Flow chart figure 6 summarizes the various stages in micropropagation of banana. Using the protocol, more than 10,000 plants are expected from a single explant at the end of 320 days as per table 6.
WORK DONE BY AUTHOR IN THE FIELD OF MICROPROPAGATION OF BANANA
Author has in depth research experience in the field of development of protocol of micropropagation of banana cultivars of commercial importance and also on the influence of ploidy and genome on culture responses which is evident by her under given research publications.
Sugandh S., Rajak K. K. and Kumar H. (2012) Diversity of genome and ploidy in banana and their effect on tissue culture responses. Res. Environ. Life Sci. 5(4), 181- 183.
Banana is one of the most important fruit crop having a wide range of ploidy and genome constitution. Most of the banana cultivars have originated from inter and intra specific hybridization of two wild diploid (2n=2x=22)
species, M. accuminata (‘A’ genome) and M. balbisiana (‘B’ genome) resulting into different genomic and ploidy levels namely AA, AAA, AAB, ABB, AAAB, AABB and ABBB. Tissue culture studies in shoot tips cultures of banana comprising different ploidy levels and having different genome structures, resulted in different forms of organogenesis. The shoot tips were cultured on MS medium supplemented with different concentrations and combinations of 2, 4-D, IAA, KIN and BAP. The effect of ploidy and genome on tissue culture responses have been found very significant. Triploids gave the best response followed by tetraploid and diploids for all tissue culture responses except somatic embryogenesis for which triploids were followed by diploids only. The genotype with more ‘A’ genomes gave better response than those with ‘B’ genome for all tissue culture responses except somatic embryogenesis as represented in figures 7(A) & 7(B).
Sugandh S., Kumari R., Sharma V. K. and Kumar
H. (2015) Isozyme analysis based genetic fidelity assessment of micropropagated banana plants. J. Applied Natural Sci. 7(2), 579 – 584.
Isozyme studies of micropropagated and mother plants of banana cvs. Matti, Ney Poovan, Kechulepa, Dwarf Cavendish, Malbhog, Champa, B.B. Battisa and FHIA-1 were done to test their genetic fidelity. The banding patterns as revealed by electrophoretic variations were evaluated with respect to isozymes of acid phosphatase, catalase, esterase and peroxidase as markers. The genetic fidelity of micropropagated plants and the relationship of the different cultivars were determined by dendrogram using numerical taxonomy and multivariate analysis system (NTSYS). A clustered dendrogram was prepared by unweighted pair group method using averages (UPGAMA) method. At 87% similarity, the micropropagated and mother plants were clustered in four groups reflecting their genomic constitution. Cvs. Matti (AA) and Dwarf Cavendish (AAA) with similar ‘A’ genome were categorized in Cluster I. Cluster II comprised of cvs. Ney Poovan (AB),
B.B. Battisa (ABB) and FHIA-1(AAAB) with genomic constitution of both ‘A’ and ‘B’ type. Cvs. Champa (AAB) and Malbhog (AAB) with similar genome were grouped in Cluster III. Cluster IV contained the cv. Kechulepa (BB) having only ‘B’ genome. However, there was no somaclonal variation among the micropropagated plants and they showed 100% genetic similarity. Thus, the isozyme studies could be a reliable marker for testing the genetic fidelity of micropropagated plants and for evaluating the diversity among the banana germplasm.

Source: Singh et al (2011)
Fig. 4: Tissue cultured plantlets at growing stage. Fig. 5 : Tissue cultured plants at fruiting stage.
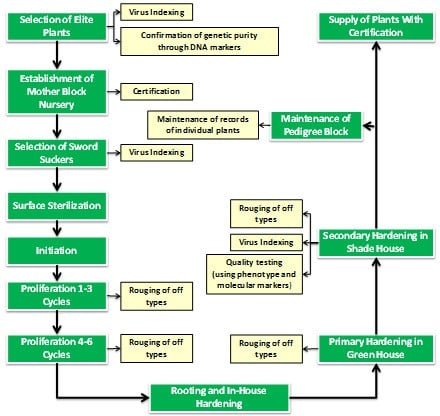
Fig. 6 : Summary of steps involved in production of quality plantlets of banana by microprogation (tissue culture).
- Sugandh S. and Kumar H. (2015) Micropropagation of Banana cv. Malbhog. The Bioscan 10(2), 647-650.
Malbhog, one of the most important and delicious local cultivar of banana in Bihar, is on verge of becoming extinct because of panama wilt and non-availability of disease free quality propagules. The culture of shoot tips taken from suckers on Murashige and Skoog (MS) medium supplemented with different concentrations and combinations of indole acetic acid (IAA) and benzyl amino purine (BAP) resulted in differentiation of
adventitious shoots. The maximum differentiation of shoots (92.05%) was observed on MS medium with 0.57 µM IAA and 17.74 ìM BAP. The number of shoots per culture was 16.75. The subculture of differentiated shoots on the same medium resulted in further differentiation (91.97%) of more than 15 shoots per culture. The in vitro developed shoots showed 100% rooting on MS medium supplemented with 4.92 ìM Indole butyric acid (IBA). The plantlets were acclimatized and field transferred. A suitable and efficient protocol for micropropagation of Malbhog cultivar of banana was
Table 5 : List of ISSR primers used for genetic fidelity testing of banana at National Research Centre for Banana (NRCB), Tiruchirapalli 620102, Tamil Nadu, India (Source: Singh et al, 2011).
|
ISSR Primer |
Nucleotide sequence |
|
UBC 807 |
5’-AGA GAG AGA GAG AGA GT-3’ |
|
UBC 808 |
5’-AGA GAG AGA GAG AGA GC-3’ |
|
UBC 811 |
5’-GAG AGA GAG AGA GAG AC-3’ |
|
UBC 812 |
5’-GAG AGA GAG AGA GAG AA-3’ |
|
UBC 818 |
5’-CAC ACA CAC ACA CAC AG-3’ |
|
UBC 830 |
5’-TGT GTG TGT GTG TGT GG-3’ |
|
UBC 834 |
5’-AGA GAG AGA GAG AGA GYT-3’ |
|
UBC 836 |
5’-AGA GAG AGA GAG AGA GYA-3’ |
|
UBC 840 |
5’-GAG AGA GAG AGA GAG AYT-3’ |
|
UBC 841 |
5’-GAG AGA GAG AGA GAG AYC-3’ |
|
UBC 842 |
5’-GAG AGA GAG AGA GAG AYG-3’ |
|
UBC 850 |
5’-GTG TGT GTG TGT GTG TYC-3’ |
|
UBC 868 |
5’-GAA GAA GAA GAA GAA GAA-3’ |
developed: figure 8.
- Sugandh S., Rajak K. K. and Kumar H. (2013) Micropropagation of Banana cv. B. B. Battisa. Biochem. Cell. Arch., 13(2), 349-354.
For banana being the most important fruit of India as whole particularly more popular in Bihar, the requirement of large numbers of quality propagules for plantation rely on the development of micropropagation protocol for important local cultivars. B.B. Battisa (ABB) is an important cultivar used as vegetable and in culinary and wine making. Shoot tips were cultured on MS medium supplemented with different concentrations of IAA (5.71, 11.42 and 22.83µM), BAP (4.43, 8.87, 17.74 and 26.61
µM) and combination of IAA (0.57, 5.71 and 11.42 µM) and BAP (8.87, 13.30 and 17.74 µM). Cultured shoot tips differentiated multiple shoots from their bases and the rate of shoot multiplication was quite high. The subculture of differentiated shoots on MS medium with
0.57 µM IAA and 17.74 µM BAP resulted in further differentiation of more than 7 shoots per culture. The in vitro developed shoots showed 100% rooting on MS medium with 4.92 µM IBA. The regenerated plantlets were transferred to a mixture of sand and farm yard manure (1:1) for acclimatization and field transfer. Thus, the present work led to the development of a suitable micropropagation protocol for cv. B.B. Battisa: figure 9.
Sugandh S., Rajak K. K., Kishore C. and Kumar
H. (2013) Micropropagation of Banana cv. Champa. Biochem. Cell. Arch. 13(2), 291-295.
Champa (AAB) is another important cultivar known for its delicious fruit used as a dessert and making pastries. Shoot tips were cultured on MS medium supplemented with different concentrations of IAA (5.71, 11.42 and
Table 6 : Success summary table of micropropagation protocol of banana (Source: Singh et al, 2011).
|
Particulars stage |
Duration (days) |
No. of plants |
|
Initiation |
25 |
1 |
|
Subculture stages 1st |
50 |
3 |
|
2nd |
75-80 |
12 |
|
3rd |
100-110 |
48 |
|
4th |
125-130 |
192 |
|
5th |
175-180 |
760 |
|
6th |
200-210 |
3,040 |
|
7th |
225-230 |
12,160 |
|
Rooting |
255-260 |
11-12,000 |
|
Primary hardening |
270-280 |
11,500-11,000* |
|
Secondary hardening |
310-320 |
10,000-10,500** |
*Success depends on the sophistication of the hardening structure
**Somaclones and off-types constitute the major discards.
22.83µM), BAP (4.43, 8.87, 17.74 and 26.61 µM) and
combination of IAA (0.57, 5.71 and 11.42 µM) and BAP (8.87, 13.30 and 17.74 µM). Cultured shoot tips differentiated multiple shoots from their bases and the rate of shoot multiplication was quite high. The subculture of differentiated shoots on MS medium with 0.57 µM IAA and 17.74 µM BAP resulted in further differentiation of more than 4 shoots per culture. The in vitro developed shoots showed 100% rooting on MS medium with 4.92 ìM IBA. The regenerated plantlets were transferred to a mixture of sand and farm yard manure (1:1) for acclimatization and field transfer. Thus, the present work led to the development of a suitable micropropagation protocol for cv. Champa: figure 10.
Sugandh S., Kumar M. and Kumar H. (2016) Plant regeneration via somatic embryogenesis from the cultured immature male floral buds of banana genotype ‘Dwarf Cavendish’. Indian J. Life Sci. 13 (2), 000-000.
Immature male floral buds of banana cv. Dwarf Cavendish were cultured on Murashige and Skoog (MS) medium supplemented with different concentrations and combinations of 2,4-D (4.52, 9.05 and 18.10 µM), IAA
(5.71, 11.42 and 22.83 µM), Kin (4.65, 9.30 and 18.60
µM) and BAP (4.43, 8.87 and 17.74 µM). The culture of the explants resulted in the generation of small spherical, friable calli. These calli showed embryogenic characteristics and sub-cultured on the same medium for further maturation. After 5 months of culture on MS basal medium, somatic embryos started to regenerate. The somatic embryos were transferred to modified MS medium for regeneration into plantlets. Among the different combinations tried, MS +9.05µM 2,4-D + 18.60µM Kin was found to be the most suitable medium for somatic embryogenesis. Thus, an efficient protocol

Fig. 7(A) : Effect of ploidy on different tissue culture responses from shoot tip culture.
Fig. 7(B) : Effect of genome (A & B) on different tissue culture response from shoot tip culture.

Fig. 8 : (a-e) : Explant showing existing
shoot elongation after 15 days of culture
on medium BM (a), Multiple shoot
8
formation after 20 days of culture on
medium BM3(b), Multiple shoot formation after 25 days of culture on medium BM11(b), Root formation after 20 days of culture on medium BM10(d), Two month-old planelet (e).


for plant regeneration via indirect somatic embryogenesis have been developed: figure 11.
CRITICAL PROBLEMS AND RESEARCHABLE ISSUES
It is evident by the literatures that the development of tissue culture protocols is a rigorous procedure that involves optimization of the various chemical, physical and environmental factors of cell and tissue growth being accomplished by growing plants outside their natural environment and growth conditions. Despite the
meticulous efforts involved in growing plants in vitro and the advances made in plant tissue culture, the application of this technique is still hampered by various physiological and developmental problems. The problems are summarized by Bairu and Kane (2011) and range from abnormal growth to increased genetic variability/instability. Of these, the points of critical attention are:
-
- Shoot-tip necrosis : It is a phenomenon whereby the apical shoot becomes brown and later dies (McCown and Sellmer, 1987). The problem of non-pathogenic
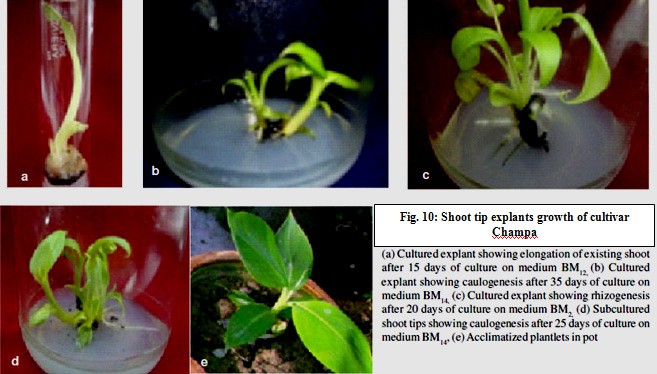

Fig. 11 : Plant regeneration from callus culture in ‘Dwarf Cavendish (a) Explant showing swelling after 20 days of culture on medium
(b) Explant showing callus formation after 40 days of culture on medium BM (c) Explant showing embryogenic callus
21
BM
14
formation after 125 days of culture on medium BM (d) & (e) Regeneration of somatic embryos into plantlets cultured on modified
17
MS medium (f) Hardening of regenerated plants in green house.
dieback or shoot-tip necrosis of in vitro cultures has been associated with various culture conditions including auxin- cytokinin interactions (Bairu and Kane, 2011).
-
- Fasciation and tissue proliferation: Fasciation is abnormal flattening of stems due to failure of the lateral branches to separate from the main stem during growth.
Some describe fasciation as fusion of organs due to deviation from normal meristematic processes while others suggest that it is the result of transformation of a single growing point into a line (Clark et al, 1993). Tissue proliferation is a disorder primarily found in micropropagated rhododendrons that is characterized by
the formation of abnormal callus-like growths, or tumors at or near the crown or base of the plant (Brand and Kiyomoto, 1992).
-
- Epigenetic changes and somaclonal variation: The distinction between epigenetic changes and somaclonal variation is that the former involves temporary changes in gene expression as a result of events such as change in DNA methylation. Such changes are temporary and often plants reverse to the normal phenotype although some changes may last longer (Brettell and Dennis, 1991) whereas somaclonal variation on the other hand involves irreversible genetic change originating in cell and tissue cultures (Larkin and Scowcroft, 1981).
- Role of phenolics and plant growth regulators in alleviating developmental problems : Limited rooting ability of tissue cultured plants is an important factor challenging the use of tissue culture. After Thimann and Went (1934) remarked that auxin promotes adventitious rooting, several discoveries were made on aspects of rooting including the root stimulating efficiency of various phenolic compounds and their relationship with auxins. The development of basal callus is one of the main physiological disorders that affect rooting competence of microplants. The superiority of metatopolins over some conventionally used cytokinins in facilitating the shoot growth has also been reported (Bairu and Kane, 2011).
More concerted research to ameliorate these upcoming problems of plant tissue culture is highly warranted in the coming years.
CONCLUSIONS
Advances in molecular biology allow for the genetic engineering of plants through the precise insertion of foreign genes from diverse biological systems. Three major breakthroughs have played major roles in the development of this transformation technology i.e. the development of shuttle vectors for harnessing the natural gene transfer capability of Agrobacterium, the methods to use these vectors for the direct transformation of re- generable explants obtained from plant organs and the development of selectable markers. The next five to ten years could be an exciting time for the banana industry as biotechnology is applied to this crop, which is highly important to the food security of so many people in developing countries and which creates income for a long chain of people engaged in the production and trade in the product. Breakthroughs can be anticipated, and hopefully these will lead to: reduced environmental impacts by chemicals, now necessarily applied in production areas; lower costs of production; improved
worker safety; and higher productivity. Moreover, it is also anticipated that whereas genetic engineering- molecular technologies offer great opportunity to develop a world that is truly food secure, we must not forget that we all – the scientific community, the international community, the multinational life science companies and the donor community together with national governments
– bear a fundamental responsibility to ensure that the developing countries can equitably share in these exciting advances that science offers in a way that is safe for their population and environment. This calls for a more open, integrated and collaborative involvement of all the stakeholders of developing country engaged in agriculture and food production. Bananas are very much a part of this opportunity.
REFERENCES
Ahmadi A, Azadfar D and Mofidabadi A J (2010) Study of inter- generic hybridization possibility between Salix aegyptica and Populus caspica to achieve new hybrids. Int. J. Plant Prod. 4(2), 143-147.
Aitken-Christie J, Kozai T and Ann Lila Smith M (1995) Automation and Environmental Control. In: Plant Tissue Culture. Kluwer Academic Publishers, Dordrecht.
Akin-Idowu P E, Ibitoye D O and Ademoyegun O T (2009) Tissue culture as a plant production technique for horticultural crops. Afr. J. Biotechnol. 8(16), 3782-3788.
Alikaridis F, Papadakis D, Pantelia K and Kephalas, T (2000) Flavonolignan production from Silybium marianum transformed and untransformed root cultures. Fitoterapia 71, 379-384.
Bachraz, D Y (1998) The role of tissue culture in agricultural Diversification. J.Food and Agricultural Research Council, Réduit, Mauritius. 1995, 96-101.
Bairu M W and Kane M E (2011) Physiological and developmental problems encountered by in vitro cultured plants. Plant Growth Regul. 63, 101–103.
Bajaj Y P S (1990) In vitro production of haploids and their use in cell genetics and plant breeding. In: Biotechnol. Agr. Forest. (ed. Bajaj Y P S), Berlin. 3-44.
Baldi A and Dixit V K (2008) Enhanced artemisinin production by cell cultures of Artemisia annua. Curr. Terends in Biotechnol. Pharmacol. 2, 341-348.
Basu S K, Datta M, Sharma M and Kumar A (2011) Haploid plant production technology in wheat and some selected higher plants. Aust. J. Crop Sci. 5(9), 1087-1093.
Bela J, Ueno K and Shetty K (1998) Control of hyperhydricity in anise (Pimpinella anisum) tissue culture by Pseudomonas spp.
J. Herbs Spices Medicinal Plants 6, 57-67.
Benson E E (2000) In vitro plant recalcitrance: An introduction. Cell.
Dev. Biol. Plant. 36, 141-148.
BioVision (2008) Modern tissue culture lab offer hope to farmers. News Letter. 10. available online at www.biovisioneastafrica.com.
Bisht R, Chaturvedi S, Srivastava R, Sharma A K and Johri BN (2009) Effect of arbuscular mycorrhizal fungi, Pseudomonas fluorescens and Rhizobium on the Growth and nutrient uptake of Dalbergia sissoo. Tropical Ecology. 50, 231-242.
Bouquet A and Terregrosa L (2003) Micropropagation of grapevine (Vitus spp.). In: Micropropagation of woody trees and fruits. (eds. Jain S M and Ishii K),The Netherlands 75, 319-352.
Brand M and Kiyomoto R (1992) Abnormal growths on micropropagated elepidote rhododendrons. Comb. Proc. Int. Plant Prop. Soc. 42, 530–534.
Brettell R I S and Dennis E S (1991) Reactivation of a silent Ac following tissue culture is associated with heritable alteration in its methylation pattern. Mol. Gen .Genet. 229, 65–372.
Brown D C W and Thorpe T A (1995) Crop improvement through tissue culture. World J. Microbiol. Biotechnol. 11, 409-415.
Brown D C W, Finstad K I and Watson E M (1995) Somatic embryogenesis in herbaceous dicots. In: In vitro Embryogenesis of Plants (ed. Thorpe T A), Kluwer Academic Publishers. Dordrecht, The Netherlands. 345-416.
Bukovinszki A, Diveki Z, Csanyi M, Palkovics L and Balazs E (2007) Engineering resistance to PVY in different potato cultivars in a marker-free transformation system using a ‘Shooter mutant’ A. tumefaciens. Plant Cell Rep. 26, 459-465.
Cassells A C and Doyle B M (2005) Pathogen and biological contamination management: the road ahead. In: Plant Cell Culture Protocols (eds. Loyola-Vargas V M and Vázquez-Flota F), Humana Press. New York, USA. 35-50.
Chadha K L and Choudhary M L (2010) Horticulture, plantation crops and organic farming for the XI five year plan (2007- 2012).Planning commission, Government of India.
Chatenet M, Delage C, Ripolles M, Irey M, Lockhart B L E and Rott P (2001) Detection of sugarcane yellow leaf virus in quarantine and production of virus-free sugarcane by apical meristem culture. Plant Disease 85(11), 1177-1180.
Chattopadhyay S, Farkya S, Srivastava A K and Bisaria V S (2002) Bioprocess Considerations for Production of Secondary Metabolites by Plant Cell Suspension Cultures. Biotechnol. Bioprocess Eng. 7, 138-149.
Chauhan H and Khurana P (2011) Use of double haploid technology for development of stable drought tolerant bread wheat (Triticum aestivum L.) transgenics. Plant Biotechnol. J. 9(3), 408-417.
Chu C C, Wang C C, Sun C S, Hsu C, Yin K C, Chu C Y and Bi F Y (1975) Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Scientia Sinica 18, 659-668.
Clark S E, Running M P and Meyerowitz E M (1993) Clavata1, a regulator of meristem and flower development in Arabidopsis. Development 119, 397–418.
Contin A, Van der Heijden R and Verpoorte R (1999) Effects of alkaloid precursor feeding and elicitation on the accumulation of secologanin in a Catharanthus roseus cell suspension culture. Plant Cell Tiss. Org. Cult. 56, 111-119.
Corredoira E, San-Jose M C, Ballester A and Vieitez A M (2004) Cryopreservation of zygotic embryo axes and somatic embryos of European chestnut. Cryo Lett. 25, 33-42.
Day J G (2004) Cryopreservation: fundamentals, mechanisms of damage on freezing/thawing and application in culture collections. Nova Hedwigia 79, 191-206.
El-Dougdoug K A and El-Shamy M M (2011) Management of viral diseases in banana using certified and virus tested plant material. Afr. J. Microbiol. Res. 5(32), 5923-5932.
Etienne-Barry D, Bertrand B and Vasquez N Etienne H (1999) Direct sowing of Coffea arabica somatic embryos mass-produced in a bioreactor and regeneration of plants. Plant Cell Rep. 19, 111- 117.
Evans D A and Bravo J E (1988) Agricultural applications of protoplast fusion. In: Plant Biotechnol.(ed. Marby T I), Austin. 51-91.
Filho A R, Dal Vesco L L, Nodari R O, Lischka R W, Müller C V and Guerra M P (2005) Tissue culture for the conservation and mass propagation of Vriesea reitzii Leme and Costa, abromelian threatened of extinction from the Brazilian Atlantic Forest. Biodivers. Conserv. 14(8), 1799-1808.
Franklin G and Lakshmi S G (2003) Agrobacterium tumefaciens– mediated transformation of eggplant (Solanum melongena L.) using root explants. Plant Cell Rep. 21, 549-554.
Fukui H, Feroj H, Ueoka T and Kyo M (1998) Formation and secretion of a new benzoquinone by hairy root cultures of Lithospermum erythrorhizon. Phytochem. 47, 10371039.
Gamborg O, Miller R and Ojima K (1968) Nutrient requirement suspensions cultures of soybean root cells. Exp. Cell Res. 50, 151-158.
Garcia R, Morán R, Somonte D, Zaldúa Z, López A and Mena C J (1999) Sweet potato (Ipomoea batatas L.) biotechnology: perspectives and progress. In: Plant Biotechnology and in vitro Biology in 21st Century. (eds. Altman A, Ziv M and Shamay I), The Netherlands. 143-146.
Garcia-Gonzales R, Quiroz K, Carrasco B and Caligari P (2010) Plant tissue culture: Current status, opportunities and challenges. Cien. Inv. Agr. 37(3), 5-30.
George E F (1993) Plant propagation by Tissue Culture. Eastern Press, Eversley.
Giri A and Narasu M (2000) Transgenic Hairy Roots: Recent Trends and Application. Biotechnol. Adv. 18, 1-22.
Guerra M P and Handro W (1988) Somatic embryogenesis and plant regeneration in embryo cultures of Euterpe edulis Mart. (Palmae). Plant Cell Rep. 7, 550-552.
Haberlandt G (1902) Kulturversuche mit isolierten Pflanzenzellen. Sitzungsber. Akad. Wiss. Wien. Math.- Naturwiss. Kl. Abt. J. 111, 69-92.
Han G Y, Wang X F, Zhang G Y and Ma Z Y (2009) Somatic embryogenesis and plant regeneration of recalcitrant cottons (Gossypium hirsutum). Afr. J. Biotechnol. 8 (3), 432-437.
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. Cryo. Lett. 25, 3-22.
Harding K (2010) Plant and algal cryopreservation: issues in genetic integrity, concepts in cryobionomics and current applications in cryobiology. Aspac J. Mol. Biol. Biotechnol. 18 (1), 151-154.
Harding K, Johnston J and Benson E E (2005) Plant and algal cell cryopreservation: issues in genetic integrity, concepts. In: Cryobionomics and current European applications: Contributing to a Sustainable Future (eds. Benett I J, Bunn E, Clarke H and McComb J A),Western Australia. 112-119.
Hellwig S, Drossard J, Twyman R M and Fischer R (2004) Plant cell cultures for the production of recombinant proteins. Nat. Biotechnol. 22, 1415- 1422.
Hinchee M A W, Corbin D R, Armstrong C L, Fry J E, Sato S S, Deboer D L, Petersen W L, Armstrong T A, Connor-Wand D Y, Layton J G and Horsch R B (1994) Plant transformation in In:
Plant Cell and Tissue Culture (eds. Vasil L K and Thorpe T A) Dordrecht: Kluwer Academic. 231-270.
Holeman D J (2009) Simple embryo culture for plant breeders: a manual of technique for the extracyion and in vitro germination of mature plant embryos with emphasis on the rose. First edition. Rose Hybridizers Association. 10.
Hu Z B and Du M (2006) Hairy Root and Its Application in Plant Genetic Engineering. J. Integr. Plant Biol. 48 (2), 121″127.
Husain M K and Anis M (2009) Rapid in vitro multiplication of Melia azedarach L. (a multipurpose woody tree). Acta Physiologiae Plantarum 31(4), 765-772.
Hussain A, Qarsh I A I, Nazir H and Ullah I (2012) Plant Tissue Culture: Current Status and Opportunities, Chapter 1, 1-28. In: Recent Advances in Plant In Vitro Culture via Intech Open Access.
Ilan A, and Workafes Woldetsadik (2011) Fruit tree nurseries and plant tissue culture. USAID- MASHAV– MoA. Minstry of Agriculture.
Jarret R L, Rodriguez W and Fernandez R (1985) Evaluation, tissue culture propagation and dissemination of ‘Saba’ and ‘Pelipita’ plantains in Costa-Rica. Scientia Horticulturae 25, 137-147.
Jayasankar S, Gray D J and Litz R E (1999) High-efficiency somatic embryogenesis and plant regeneration from suspension cultures of grapevine. Plant Cell Rep. 18, 533-537.
Jeong G A and Park D H (2007) Enhanced secondary metabolite biosynthesis by elicitation in transformed plant root system. Appl. Biochem. Biotechnol. 130, 436-446.
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plant Res. 3(13), 1222-1239.
Khan T, Krupadanam D and Anwar Y (2008) The role of phytohormone on the production of berberine in the calli culture of an endangered medicinal plant, turmeric (Coscinium fenustratum L.). Afr. J. Biotechnol. 7, 3244-3246.
Kim J S, Lee S Y and Park S U (2008) Resveratol production in hairy root culture of peanut, Arachys hypogaea L. transformed with differet Agrobacterium rhizogenes strains. Afr. J. Biotechnol. 7, 3788-3790.
Kim O T, Bang K H, Shin Y S, Lee M J, Jang S J, Hyun D Y, Kim Y C, Senong N S, Cha S W and Hwang B (2007) Enhanced production of asiaticoside from hairy root cultures of Centella asitica (L.) Urban elicited by methyl jasmonate. Plant Cell Rep. 26, 1914-1949.
Kumar A, Sharma D, Tiwari A, Jaiswal J P, Singh N K and Sood S (2016) Genotyping-by-Sequencing Analysis for Determining Population Structure of Finger Millet Germplasm of Diverse Origins. The Plant Genome. 9(2), 1-15.
Lakshmanan P, Lee C L and Goh C J (1996). In vitro propagation of commercial orchids: An assessment of current methodologies and development of a novel approach – thin section culture system. J. Orchid Soc. Indian 10, 31-41.
Lakshmanan P, Loh C S and Goh C J (1995). An in vitro method for rapid regeneration of a monopodial orchid hybrid Aranda deborah using thin section culture. Plant Cell Rep. 14, 510-514.
Larkin P and Scowcroft W (1981) Somaclonal variation–a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60, 197–214.
Lee J M (2004). Plant cell culture and its applications. In: R. M
Goodman (ed), Encyclopaedia of Plant and Crop Sciences,
Marcel Dekker, USA. 931-933.
Lee S Y, Cho S J, Park M H, Kim YK, Choi J I, Park S U (2007) Growth and rutin production in hairy root culture of buck weed (Fagopyruum esculentum). Prep. Biochem. Biotechnol. 37, 239- 246.
Lee-Parsons C W T and Rogce A J (2006) Precursor limitations in methyl jasmonate-induced Catharanthus roseus cell cultures. Plant Cell Rep. 25, 607-612.
Leon J, Rojo E and Sanchez-Serrano J J (2001) Wound signalling in plants. J. Exp. Bot. 52, 1- 9.
Lila K M (2005) Valuable secondary products from in vitro culture,
Secondary Products In Vitro. CRC Press LLC.
Linsmaier E M and Skoog F (1975) Organic growth factor requirements of tobacco tissue cultures. Physiologia Plantarum 18, 100.
Liu Z Y, Chen P D, Pei G Z, Wang Y N, Qin B X and Wang S L (1988) Transfer of Haynaldia villosa chromosomes into Triticum aestivum. Proceeding of the 7th International Wheat Genetics Sumposium, Cambridge, UK. 355-361.
Lovato P E, Gianinazzi-Pearson V, Trouvelot A and Gianinazzi S (1996). The state of mycorrhizas and micropropagation. Adv. Hort. Sci. 10, 46-52.
Ma S S and Shii C T (1974) Growing banana plantlets from adventitious buds. J.Chinese Society of Horticultural Sci. 20, 6-12.
Ma S S, Shii C T and Wang S O (1978) Regeneration of banana plants from shoot meristem tips and inflorescence sections in vitro. In: Abstract of the 20th Int. Hort. Congress, Sydney, Australia, Abstract No. 1639.
Marana J P, Miglioranza E and De Faria R T (2009) In vitro establishment of Jacaratia spinosa (Aubl.) ADC. Semina- Ciencias Agrarias 30(2), 271-274.
Marconi P L, Selten L M, Cslcena E N, Alvarez M A and Pitta- Alvarez S I (2008) Changes in growth and tropane alkaloid production in long term culture of hairy roots of Brugmansia candida. Elect. J. Integrative Biosci. 3, 38-44.
Marino G and Battistini S (1990) Leaf-callus growth, shoot regeneration and somaclonal variation in Actinidia deliciosa: effect of media pH. Acta Horticulturae 280, 37-44.
Maynard C, Xiang Z, Bickel S and Powell W (1998) Using genetic engineering to help save American chestnut: a progress report. J. Am Chestnut Found. 12, 40-56.
McCown B H and Sellmer J C (1987) General media and vessels suitable for woody plant culture. In: Cell and tissue culture in forestry: General principles and biotechnology, (eds. Bonga J M and Durzan), Vol. 1. Martinus Nijhoff Publishers, Dordrecht, pp 4–16.
Mehrotra S, Kukreja A K, Khanuja S P S and Mishra B N (2008) Genetic transformation studies and scale up of hairy root culture of Glycyrrhiza glabra in bioreactor. Elect. J. Biotech., 11, 717- 728.
Mohan N, Nikdad S and Singh G (2011) Studies on seed germination and embryo culture of Jatropha curcas L. under in vitro conditions. Research Article, Biotechnol, Bioinf, Bioeng. 1(2), 187-194.
Monier C, Bossis E, Chabanet C and Samson R (1998) Different bacteria can enhance the micropropagation response of Cotoneaster lacteus (Rosaceae). J. Appl. Microbiol. 5, 1047-
1055.
Moreno P R H, Van der Heijden R and Verpoorte R (1993) Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep. J. 12, 702- 705.
Morini S, D’Onofrio C, Bellocchi G and Fisichella M (2000) Effect of 2, 4-D and light quality on callus production and differentiation from in vitro cultured quince leaves. Plant Cell Tiss. Organ Cult. 63, 47-55.
Morrison R A and Evans D A (1998) Haploid plants from tissue culture: New plant varieties in a shortened time frame. Nat. Biotechnol. 6, 684-690.
Mostageer A and Elshihy O M (2003) Establishment of salt tolerant somatic hybrid through protoplast fusion between rice and ditch reed. Arab. J. Biotech. 6(1), 01-12.
Motomura T, Hidaka T, Akihama T and Omura M (1997) Protoplast fusion for production of hybrid plants between citrus and its related genera. J. Japan. Soc. Hort. Sci. 65, 685- 692.
Mtui G Y S (2011) Status of biotechnology in Eastern and Central Africa. Biotechnology and Molecular Biology. 6(9), 183-198. Available online at http://www.academicjournals.org/BMBR.
Murashige T (1974) Plant propagation through tissue culture. Ann.
Rev. Plant Physiol. 25, 135-166.
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 15, 473-497.
Murthy H N, Dijkstra C, Anthony P, White D A, Davey M R, Powers J B, Hahn E J and Paek K Y (2008) Establishemnt of Withania somnifera hairy root cultures for the production of Withanoloid
A. Integ. Plant Biol. J. 50, 915-981.
Novak F J, Afza R, Van Duran M, Perea-Dallas M, Conger B V and Xiaolang T (1989) Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA, AAA) and cooking (ABB) bananas. BioTechnology, 7, 154-159.
Okere A U and Adegey A (2011) In vitro propagation of an endangered medicinal timber species Khaya grandifoliola C. Dc. Afr. J. Biotechnol. 10(17), 3335-3339.
Paek K Y, Hahn E J and Son S H (2001) Application of bioreactors for largescale micropropagation systems of plants. Cell. Dev. Biol. Plant. 37, 149-157.
Palazon J, Pinol M T, Cusido R M, Morales C and Bonfill M (1997) Application of transformed root technology to the production of bioactive metabolites. Recent Res. Dev. Plant Physiol. 1, 125- 143.
Park S U and Lee S Y (2009) Anthraquinone production by hairy root culture of Rubia akane Nakai : Influence of media and auxin treatment. Sci. Res. Essay. J. 4, 690-693.
Park Y S, Barrett J D and Bonga J M (1998) Application of somatic embryogenesis in high value clonal forestry: development, genetic control and stability of cryopreserved clones. Cell. Dev. Biol. Plant. 34, 231-239.
Payne G F, Shuler M L and Brodelius P (1987) Plant cell culture. In: Large Scale Cell Culture Technology (ed. Lydensen B K), Hanser Publishers, New York, USA. 193-229.
Pistelli L, Giovannini A, Ruffoni B, Bertoli A and Pistelli L (2010) Hairy Root Cultures for Secondary Metabolites Production. ISBN: 978-1-4419-7346-7.
Rahnama H, Hasanloo T, Shams M R and Sepehrifar R (2008) Silymarin production by hairy root culture of Silybium marianum (L.) Gaertn. Iranian Biotechnol. J. 6, 113-118.
Rai M K (2001) Current advances in mycorrhization in micropropagation. In vitro: Cell. Dev. Biol. Plant. 37, 158-167.
Rao S R and Ravishankar G A (2002) Plant tissue cultures; chemical factories of secondary metabolites. Biotechnol. Adv. 20, 101- 153.
Roat C and Ramawat K G (2009) Elicitor induced accumulation of stilbenes in cell suspension cultures of Cayratia trifoliata (L.) Domin. Plant Biotechnol. Rep. J. 3, 135138.
Sahay N S and Varma A (1999). Piriformospora indica: a new biological hardening tool for micropropagated plants. FEMS Microbiol. Lett. 181, 297-302.
Saini R and Jaiwal P K (2002) Age, position in mother seedling, orientation, and polarity of the epicotyl segments of blackgram (Vigna mungo L. Hepper) determines its morphogenic response. Plant Sci. 163 (1), 101-109.
Sasson A (1993) Biotechnologies in developing countries, present and future, Vol.1. Paris: United Nations Educational, Scientific and Cultural Organization.
Schenk R U and Hildebrant A C (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Canadian J.Bot. 50, 199-204.
Sengar R S, Chaudhary R and Tyagi S K (2010) Present status and scope of floriculture developed through different biological tools. Res J. Agri. Sci. 1(4), 306-314.
Shalaka D K and Sandhya P (2009) Micropropagation and organogenesis in Adhatoda vasica for the estimation of vasine. Pharm. Mag. 5, 539-363.
Sharma A K, Wahab S and Srivastava R (2010) Agriculture Diversification: Problems and Perspectives. I.K. International Publishing House Pvt. Ltd., New Delhi. 210.
Shrivastava N, Patel T and Srivastava A (2006) Biosynthetic potential of in vitro grown callus cells of Cassia senna L. var. senna. Curr. Sci. J. 90, 1472-1473.
Siahsar B, Rahimi M, Tavassoli A and Raissi A S (2011) Application of Biotechnology in Production of Medicinal Plants. J. Agric. Environ. Sci. 11(3), 439-444.
Sinclair T R, Purcell L C and Sneller C H (2004) Crop transformation and the challenge to increase yield potential. Trend Plant Sci. 9, 70-75.
Singh G and Shetty S (2011) Impact of Tissue Culture on Agriculture in India. Society for Applied Applied Biotech. 1(2), 147-188.
Singh H P, Uma S, Selvarajan R and Karihaloo J L (2011) Micropropagation for Production of Quality Banana Planting Material in Asia-Pacific. Asia-Pacific Consortium on Agricultural Biotechnology (APCoAB), New Delhi, India. 92.
Singh K K and Gurung B (2009) In vitro propagation of R. maddeni Hook. F. an endangered Rhododendron species of Sikkim Himalaya. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 37(1), 79-83.
Singh R B (1992) Current status and future prospects of plant biotechnologies in developing countries in Asia. In: Sasson A, Costarini, editors. Plant Biotechnologies for Developing Countries. London. 141-162.
Skoog F and Miller C O (1957) Chemical regulation of growth and
organ formation in plant tissue cultures in vitro. Symp. Soc. Exp. Biol. 11, 118-131.
Skrzypek Z and Wysokinsku H (2003) Sterols and titerpenes in cell cultures of Hyssopus officinalis L. Ver Lag der Zeitschrift fur Naturforschung. D. 312.
Srivastava R, Mehta C M and Sharma A K (2011) Fusarium pallidoroseum – A new biofertilizer responsible for enhancing plant growth in different crops. Int. Res. J. Microbiol. 2, 192- 199.
Srivastava S and Srivastava AK (2007) Hairy root culture for mass- production of highvalue secondary metabolites. Crit. Rev. Biotechnol. 27(1), 29-43.
Sudherson C S, Manuel J and Al-Sabah (2008) Haploid plant production from pollen grains of sturt’s desert pea via somatic embryogenesis. Am-Euras. Sci. Res. 3(1), 44-47.
Sujanya S, Poornasri D B and Sai I (2008) In vitro production of azadirachtin from cell suspension cultures of Azadirachta indica. Biosci. J. 33, 113-120.
Suman S and Kumar H (2016) Plant regeneration via somatic embryogenesis from the cultured immature male floral buds of banana genotype ‘Dwarf Cavendish’. Natl. J. Life Sci. 13(2), 00- 00 (In Press).
Suman S, Kumar R, Sharma V K and Kumar H (2015) Isozyme analysis based genetic fidelity assessment of micrpropagated banana plants. J. Appl. Natural Sci. 7(2), 579-584.
Suman S, Rajak K K and Kumar H (2012) Diversity of genome and ploidy in banana and their effect on tissue culture responses. Res. Environ. Life Sci. 5(4), 181-183.
Sylvia D (1998). The Promise (and obstacles) of AMF inoculation.
p.152. In: Arbuscular mycorrhizas in sustainable soil-plant systems (eds.Gianinnazzi S and Schuepp H), Report of 1997 activities, Cost Action 821, Iceland.
Tabata M and Fujita Y (1985) Production of shikonin by plant cell cultures. In: Biotechnology in Plant Science. Relevance to Agriculture in the Eighties, (eds. Zaitlin M, Day P and Hollaender A), Acedemic Press, San Diego, USA. 207-218.
Takayama S (1991). Mass production of plants through shake-and bioreactor-culture techniques. In: Biotechnology in Agriculture and Forestry High-Tech and Micropropagation 1. (ed. Bajaj Y P S), Springer-Verlag, Berlin, 17, 495-515.
Tanaka N, Takao M and Matsumoto T (2004) Vincamine production in multiple shoot culture derived from hairy roots of Vinca major. Plant Cell Tiss. Org. Cult. J. 41, 61-64.
Thimann KV and Went F W (1934) On the chemical nature of the root forming hormone. Pro. Kon. Akad. Wetensch. 37, 456–459.
Thorpe T (2007) History of plant tissue culture. J. Mol. Microbial Biotechnol. 37, 169-180.
Tilkat E, Onay A, Yildirim H and Ayaz E (2009) Direct plant regneration from mature leaf explants of pistachio, Pistacia vera L. Scientia Hort. 121(3), 361-365,
Ting I P (1982) Plant Physiology. Addison-Wesleyn Reading, Massachusetts. 642.
Tiwari K K, Trivedi M, Guang Z C, Guo G Q and Zheng G C (2007) Genetic transformation of Gentiana macrophylla with Agrobacterium rhizogenes: growth and production of secoiridoid glucoside gentiopicroside in transformed hairy root cultures. Plant Cell Rep. J. 26, 199-210.
Toriyama K, Hinata K and Kameya T (1987) Production of somatic hybrid plants, “Brassicomoricandia”, through protoplast fusion between Moricandia arvensis and Brassica oleracea. Plant Sci. 48(2), 123-128.
Tyagi R K, Agrawal A, Mahalakshmi C, Hussain Z and Tyagi H (2007) Low-cost media for in vitro conservation of turmeric (Curcuma longa L.) and genetic stability assessment using RAPD markers. In Vitro Cell. Develop. Biol. Plant. 43, 51-58.
Umamaheswai A and Lalitha V (2007) In vitro effect of various growth hormones in Capsicum annum L. on the callus induction and production of Capsiacin. Plant Sci. J. 2, 545-551.
Vasane R, Shailesh R M and Kothari (2006) Optimization of secondary hardening process of banana plantlets (Musa paradisiaca L. var. Grand Naine). Indian J. Biotechnol. 5, 394-399.
Verma P C, Singh D, Rahman L, Gupta M M and Banerjee S (2002) In vitro studies in Plumbago zeylanica : rapid micropropagation and establishment of higher plumbagin yeilding hairy root cultures. Plant Physiol. J. 159, 547-552.
Vijayasree N, Udayasri P, Aswani KY, Ravi B B, Phani K Y and Vijay V M (2010) Advancements in the Production of Secondary Metabolites. J. Nat. Prod. 3, 112-123.
Vuylsteke D and De Langhe E (1985) Feasibility of in vitro propagation of bananas and plantains. Tropical Agriculture (Trinidad). 62, 323-328.
Wagiah M E, Alam G, Wiryowidagdo S and Attia K (2008) Imporved production of the indole alkaloid cathin-6-one from cell suspension cultures of Brucea javanica (L.). Merr. Sci. Technol.
J. 1, 1-6.
Wambugu F M and Kiome R M (2001) The benefits of biotechnology for small scale banana producers in Kenya. ISAAA, Briefts No.
22.ISAAA Ithaca, NY. The International Services for the Acquisition of Agribiotech Application
Wang H Q, Yu J T and Zhong J J (1999) Significant improvement of taxane production in suspension cultures of Taxus chinensis by sucrose feeding strategy. Process Biochem. J. 35, 479-483.
Williams R R (1995) The chemical micro-environment. Kluwer Academic, Netherlands. 405-439.
Xiangqian L I, Krasnyanski F S and Schuyler S K (2002) Somatic embryogenesis, secondary somatic embryogenesis and shoot organogenesis in Rosa. Plant Physiol. 159, 313-319.
Xu H, Kim Y K, Suh S Y, Udin M R, Lee S Y and Park S U (2008) Deoursin production from hariy root culture of Angelica gigas. Korea Soc. Appl. Biol. Chem. J. 51, 349-351.
Yesil-Celiktas O, Gurel A and Vardar-Sukan F (2010) Large scale cultivation of plant cell and tissue culture in bioreactors. Transworld Res. Network. 1- 54.
Yeung E C, Thorpe T A and Jensen C J (1981) In vitro fertilization and embryo culture in plant tissue culture: Methods and Applications in Agriculture, ed. Thorpe, T.A. Academic Press, New York. 253-271.
Zhao J, Zhu W and Hu Q (2001) Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzyme Microbiol. Technol. J. 28, 673-681.
Zhong J J, Chen F and Hu W W (1999) High density cultivation of Panax notoginseng cells in stirred tank bioreactors for the production of ginseng biomass and ginseng saponin. Process Biochem. J. 35, 491-496.
